Functional map of arrestin-1 at single amino acid resolution
- PMID: 24449856
- PMCID: PMC3918777
- DOI: 10.1073/pnas.1319402111
Functional map of arrestin-1 at single amino acid resolution
Abstract
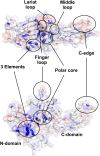
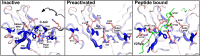
Arrestins function as adapter proteins that mediate G protein-coupled receptor (GPCR) desensitization, internalization, and additional rounds of signaling. Here we have compared binding of the GPCR rhodopsin to 403 mutants of arrestin-1 covering its complete sequence. This comprehensive and unbiased mutagenesis approach provides a functional dimension to the crystal structures of inactive, preactivated p44 and phosphopeptide-bound arrestins and will guide our understanding of arrestin-GPCR complexes. The presented functional map quantitatively connects critical interactions in the polar core and along the C tail of arrestin. A series of amino acids (Phe375, Phe377, Phe380, and Arg382) anchor the C tail in a position that blocks binding of the receptor. Interaction of phosphates in the rhodopsin C terminus with Arg29 controls a C-tail exchange mechanism in which the C tail of arrestin is released and exposes several charged amino acids (Lys14, Lys15, Arg18, Lys20, Lys110, and Lys300) for binding of the phosphorylated receptor C terminus. In addition to this arrestin phosphosensor, our data reveal several patches of amino acids in the finger (Gln69 and Asp73-Met75) and the lariat loops (L249-S252 and Y254) that can act as direct binding interfaces. A stretch of amino acids at the edge of the C domain (Trp194-Ser199, Gly337-Gly340, Thr343, and Thr345) could act as membrane anchor, binding interface for a second rhodopsin, or rearrange closer to the central loops upon complex formation. We discuss these interfaces in the context of experimentally guided docking between the crystal structures of arrestin and light-activated rhodopsin.
Keywords: cell signaling; membrane receptor; protein engineering; scanning mutagenesis; visual system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Deupi X, Standfuss J. Structural insights into agonist-induced activation of G-protein-coupled receptors. Curr Opin Struct Biol. 2011;21(4):541–551. - PubMed
-
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308(5721):512–517. - PubMed
-
- Choe H-W, et al. Crystal structure of metarhodopsin II. Nature. 2011;471(7340):651–655. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

