Development of pattern vision following early and extended blindness
- PMID: 24449865
- PMCID: PMC3918801
- DOI: 10.1073/pnas.1311041111
Development of pattern vision following early and extended blindness
Abstract
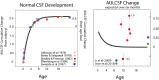
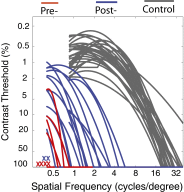
Visual plasticity peaks during early critical periods of normal visual development. Studies in animals and humans provide converging evidence that gains in visual function are minimal and deficits are most severe when visual deprivation persists beyond the critical period. Here we demonstrate visual development in a unique sample of patients who experienced extended early-onset blindness (beginning before 1 y of age and lasting 8-17 y) before removal of bilateral cataracts. These patients show surprising improvements in contrast sensitivity, an assay of basic spatial vision. We find that contrast sensitivity development is independent of the age of sight onset and that individual rates of improvement can exceed those exhibited by normally developing infants. These results reveal that the visual system can retain considerable plasticity, even after early blindness that extends beyond critical periods.
Keywords: brain plasticity; childhood blindness; sensitive periods; sight restoration; visual impairment.
Conflict of interest statement
Conflict of interest statement: L.A.L., P.J.B., and M.D. declare intellectual property interest in contrast sensitivity testing (US 7,938,538 and US 61/644,889), and equity interest in a venture, Adaptive Sensory Technology, to commercialize such testing.
Figures


References
-
- Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965;28(6):1029–1040. - PubMed
-
- Movshon JA, Kiorpes L. In: Early Visual Development, Normal and Abnormal. Simons K, editor. New York: Oxford Univ Press; 1993. pp. 296–305.
-
- Braastad BO, Heggelund P. Development of spatial receptive-field organization and orientation selectivity in kitten striate cortex. J Neurophysiol. 1985;53(5):1158–1178. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

