Paternally induced transgenerational inheritance of susceptibility to diabetes in mammals
- PMID: 24449870
- PMCID: PMC3918818
- DOI: 10.1073/pnas.1321195111
Paternally induced transgenerational inheritance of susceptibility to diabetes in mammals
Abstract
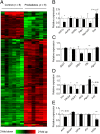
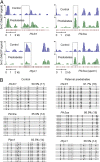
The global prevalence of prediabetes and type 2 diabetes (T2D) is increasing, and it is contributing to the susceptibility to diabetes and its related epidemic in offspring. Although the impacts of paternal impaired fasting blood glucose and glucose intolerance on the metabolism of offspring have been well established, the exact molecular and mechanistic basis that mediates these impacts remains largely unclear. Here we show that paternal prediabetes increases the susceptibility to diabetes in offspring through gametic epigenetic alterations. In our findings, paternal prediabetes led to glucose intolerance and insulin resistance in offspring. Relative to controls, offspring of prediabetic fathers exhibited altered gene expression patterns in the pancreatic islets, with down-regulation of several genes involved in glucose metabolism and insulin signaling pathways. Epigenomic profiling of offspring pancreatic islets revealed numerous changes in cytosine methylation depending on paternal prediabetes, including reproducible changes in methylation over several insulin signaling genes. Paternal prediabetes altered overall methylome patterns in sperm, with a large portion of differentially methylated genes overlapping with that of pancreatic islets in offspring. Our study uniquely revealed that prediabetes can be inherited transgenerationally through the mammalian germ line by an epigenetic mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

