Variants of mouse DNA polymerase κ reveal a mechanism of efficient and accurate translesion synthesis past a benzo[a]pyrene dG adduct
- PMID: 24449898
- PMCID: PMC3918839
- DOI: 10.1073/pnas.1324168111
Variants of mouse DNA polymerase κ reveal a mechanism of efficient and accurate translesion synthesis past a benzo[a]pyrene dG adduct
Abstract
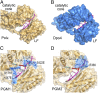
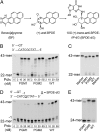
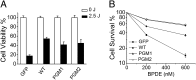
DNA polymerase κ (Polκ) is the only known Y-family DNA polymerase that bypasses the 10S (+)-trans-anti-benzo[a]pyrene diol epoxide (BPDE)-N(2)-deoxyguanine adducts efficiently and accurately. The unique features of Polκ, a large structure gap between the catalytic core and little finger domain and a 90-residue addition at the N terminus known as the N-clasp, may give rise to its special translesion capability. We designed and constructed two mouse Polκ variants, which have reduced gap size on both sides [Polκ Gap Mutant (PGM) 1] or one side flanking the template base (PGM2). These Polκ variants are nearly as efficient as WT in normal DNA synthesis, albeit with reduced accuracy. However, PGM1 is strongly blocked by the 10S (+)-trans-anti-BPDE-N(2)-dG lesion. Steady-state kinetic measurements reveal a significant reduction in efficiency of dCTP incorporation opposite the lesion by PGM1 and a moderate reduction by PGM2. Consistently, Polκ-deficient cells stably complemented with PGM1 GFP-Polκ remained hypersensitive to BPDE treatment, and complementation with WT or PGM2 GFP-Polκ restored BPDE resistance. Furthermore, deletion of the first 51 residues of the N-clasp in mouse Polκ (mPolκ(52-516)) leads to reduced polymerization activity, and the mutant PGM2(52-516) but not PGM1(52-516) can partially compensate the N-terminal deletion and restore the catalytic activity on normal DNA. However, neither WT nor PGM2 mPolκ(52-516) retains BPDE bypass activity. We conclude that the structural gap physically accommodates the bulky aromatic adduct and the N-clasp is essential for the structural integrity and flexibility of Polκ during translesion synthesis.
Keywords: polycyclic aromatic hydrocarbons; translesion DNA synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Effects of the N terminus of mouse DNA polymerase κ on the bypass of a guanine-benzo[a]pyrenyl adduct.J Biochem. 2016 Apr;159(4):471-9. doi: 10.1093/jb/mvv118. Epub 2015 Dec 3. J Biochem. 2016. PMID: 26634445 Free PMC article.
-
Structure and mechanism of error-free replication past the major benzo[a]pyrene adduct by human DNA polymerase κ.Nucleic Acids Res. 2016 Jun 2;44(10):4957-67. doi: 10.1093/nar/gkw204. Epub 2016 Mar 31. Nucleic Acids Res. 2016. PMID: 27034468 Free PMC article.
-
Activities of human DNA polymerase kappa in response to the major benzo[a]pyrene DNA adduct: error-free lesion bypass and extension synthesis from opposite the lesion.DNA Repair (Amst). 2002 Jul 17;1(7):559-69. doi: 10.1016/s1568-7864(02)00055-1. DNA Repair (Amst). 2002. PMID: 12509229
-
Structural basis of accurate replication beyond a bulky major benzo[a]pyrene adduct by human DNA polymerase kappa.DNA Repair (Amst). 2017 Jan;49:43-50. doi: 10.1016/j.dnarep.2016.11.001. Epub 2016 Nov 19. DNA Repair (Amst). 2017. PMID: 27894903
-
Phenylalanine 171 is a molecular brake for translesion synthesis across benzo[a]pyrene-guanine adducts by human DNA polymerase kappa.Mutat Res. 2011 Jan 10;718(1-2):10-7. doi: 10.1016/j.mrgentox.2010.11.002. Epub 2010 Nov 13. Mutat Res. 2011. PMID: 21078407
Cited by
-
Structural and Molecular Kinetic Features of Activities of DNA Polymerases.Int J Mol Sci. 2022 Jun 7;23(12):6373. doi: 10.3390/ijms23126373. Int J Mol Sci. 2022. PMID: 35742812 Free PMC article. Review.
-
Effects of the N terminus of mouse DNA polymerase κ on the bypass of a guanine-benzo[a]pyrenyl adduct.J Biochem. 2016 Apr;159(4):471-9. doi: 10.1093/jb/mvv118. Epub 2015 Dec 3. J Biochem. 2016. PMID: 26634445 Free PMC article.
-
Effects of the Y432S Cancer-Associated Variant on the Reaction Mechanism of Human DNA Polymerase κ.J Chem Inf Model. 2024 May 27;64(10):4231-4249. doi: 10.1021/acs.jcim.4c00339. Epub 2024 May 8. J Chem Inf Model. 2024. PMID: 38717969 Free PMC article.
-
Translesion Synthesis of the N(2)-2'-Deoxyguanosine Adduct of the Dietary Mutagen IQ in Human Cells: Error-Free Replication by DNA Polymerase κ and Mutagenic Bypass by DNA Polymerases η, ζ, and Rev1.Chem Res Toxicol. 2016 Sep 19;29(9):1549-59. doi: 10.1021/acs.chemrestox.6b00221. Epub 2016 Aug 17. Chem Res Toxicol. 2016. PMID: 27490094 Free PMC article.
-
Mutational analysis of the C8-guanine adduct of the environmental carcinogen 3-nitrobenzanthrone in human cells: critical roles of DNA polymerases η and κ and Rev1 in error-prone translesion synthesis.Biochemistry. 2014 Aug 19;53(32):5323-31. doi: 10.1021/bi5007805. Epub 2014 Aug 6. Biochemistry. 2014. PMID: 25080294 Free PMC article.
References
-
- Brookes P, Osborne MR. Mutation in mammalian cells by stereoisomers of anti-benzo[a] pyrene-diolepoxide in relation to the extent and nature of the DNA reaction products. Carcinogenesis. 1982;3(10):1223–1226. - PubMed
-
- Geacintov NE, et al. NMR solution structures of stereoisometric covalent polycyclic aromatic carcinogen-DNA adduct: Principles, patterns, and diversity. Chem Res Toxicol. 1997;10(2):111–146. - PubMed
-
- Friedberg E, et al. DNA Repair and Mutagenesis. 2nd Ed. Washington, DC: ASM; 2006.
-
- Ohmori H, et al. The Y-family of DNA polymerases. Mol Cell. 2001;8(1):7–8. - PubMed
-
- Yang W. Damage repair DNA polymerases Y. Curr Opin Struct Biol. 2003;13(1):23–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

