Comprehensive structural model of the mechanochemical cycle of a mitotic motor highlights molecular adaptations in the kinesin family
- PMID: 24449904
- PMCID: PMC3918802
- DOI: 10.1073/pnas.1319848111
Comprehensive structural model of the mechanochemical cycle of a mitotic motor highlights molecular adaptations in the kinesin family
Abstract
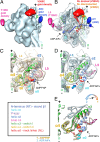
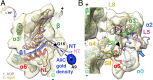
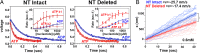
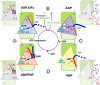
Kinesins are responsible for a wide variety of microtubule-based, ATP-dependent functions. Their motor domain drives these activities, but the molecular adaptations that specify these diverse and essential cellular activities are poorly understood. It has been assumed that the first identified kinesin--the transport motor kinesin-1--is the mechanistic paradigm for the entire superfamily, but accumulating evidence suggests otherwise. To address the deficits in our understanding of the molecular basis of functional divergence within the kinesin superfamily, we studied kinesin-5s, which are essential mitotic motors whose inhibition blocks cell division. Using cryo-electron microscopy and determination of structure at subnanometer resolution, we have visualized conformations of microtubule-bound human kinesin-5 motor domain at successive steps in its ATPase cycle. After ATP hydrolysis, nucleotide-dependent conformational changes in the active site are allosterically propagated into rotations of the motor domain and uncurling of the drug-binding loop L5. In addition, the mechanical neck-linker element that is crucial for motor stepping undergoes discrete, ordered displacements. We also observed large reorientations of the motor N terminus that indicate its importance for kinesin-5 function through control of neck-linker conformation. A kinesin-5 mutant lacking this N terminus is enzymatically active, and ATP-dependent neck-linker movement and motility are defective, although not ablated. All these aspects of kinesin-5 mechanochemistry are distinct from kinesin-1. Our findings directly demonstrate the regulatory role of the kinesin-5 N terminus in collaboration with the motor's structured neck-linker and highlight the multiple adaptations within kinesin motor domains that tune their mechanochemistries according to distinct functional requirements.
Keywords: cancer; macromolecular assemblies; mitosis; molecular motors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The structural basis of force generation by the mitotic motor kinesin-5.J Biol Chem. 2012 Dec 28;287(53):44654-66. doi: 10.1074/jbc.M112.404228. Epub 2012 Nov 7. J Biol Chem. 2012. PMID: 23135273 Free PMC article.
-
Conserved mechanisms of microtubule-stimulated ADP release, ATP binding, and force generation in transport kinesins.Elife. 2014 Sep 10;3:e03680. doi: 10.7554/eLife.03680. Elife. 2014. PMID: 25209998 Free PMC article.
-
The kinesin-5 tail domain directly modulates the mechanochemical cycle of the motor domain for anti-parallel microtubule sliding.Elife. 2020 Jan 20;9:e51131. doi: 10.7554/eLife.51131. Elife. 2020. PMID: 31958056 Free PMC article.
-
Kinesin, 30 years later: Recent insights from structural studies.Protein Sci. 2015 Jul;24(7):1047-56. doi: 10.1002/pro.2697. Epub 2015 Jun 11. Protein Sci. 2015. PMID: 25975756 Free PMC article. Review.
-
Kinesin: a molecular motor with a spring in its step.Proc Biol Sci. 2002 Nov 22;269(1507):2363-71. doi: 10.1098/rspb.2002.2117. Proc Biol Sci. 2002. PMID: 12495505 Free PMC article. Review.
Cited by
-
Nucleotide-free structures of KIF20A illuminate atypical mechanochemistry in this kinesin-6.Open Biol. 2023 Sep;13(9):230122. doi: 10.1098/rsob.230122. Epub 2023 Sep 20. Open Biol. 2023. PMID: 37726093 Free PMC article.
-
Kinesin-5 is a microtubule polymerase.Nat Commun. 2015 Oct 6;6:8160. doi: 10.1038/ncomms9160. Nat Commun. 2015. PMID: 26437877 Free PMC article.
-
Visualizing microtubule structural transitions and interactions with associated proteins.Curr Opin Struct Biol. 2016 Apr;37:90-6. doi: 10.1016/j.sbi.2015.12.009. Epub 2016 Jan 21. Curr Opin Struct Biol. 2016. PMID: 26803284 Free PMC article. Review.
-
New insights into the mechanochemical coupling mechanism of kinesin-microtubule complexes from their high-resolution structures.Biochem Soc Trans. 2023 Aug 31;51(4):1505-1520. doi: 10.1042/BST20221238. Biochem Soc Trans. 2023. PMID: 37560910 Free PMC article. Review.
-
The yeast kinesin-5 Cin8 interacts with the microtubule in a noncanonical manner.J Biol Chem. 2017 Sep 1;292(35):14680-14694. doi: 10.1074/jbc.M117.797662. Epub 2017 Jul 12. J Biol Chem. 2017. PMID: 28701465 Free PMC article.
References
-
- Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10(10):682–696. - PubMed
-
- Vale RD, Milligan RA. The way things move: Looking under the hood of molecular motor proteins. Science. 2000;288(5463):88–95. - PubMed
-
- Goulet A, Moores C. New insights into the mechanism of force generation by kinesin-5 molecular motors. Int Rev Cell Mol Biol. 2013;304:419–466. - PubMed
-
- Kapitein LC, et al. The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature. 2005;435(7038):114–118. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

