Membrane adhesion dictates Golgi stacking and cisternal morphology
- PMID: 24449908
- PMCID: PMC3918774
- DOI: 10.1073/pnas.1323895111
Membrane adhesion dictates Golgi stacking and cisternal morphology
Abstract
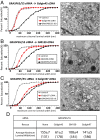
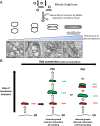
Two classes of proteins that bind to each other and to Golgi membranes have been implicated in the adhesion of Golgi cisternae to each other to form their characteristic stacks: Golgi reassembly and stacking proteins 55 and 65 (GRASP55 and GRASP65) and Golgin of 45 kDa and Golgi matrix protein of 130 kDa. We report here that efficient stacking occurs in the absence of GRASP65/55 when either Golgin is overexpressed, as judged by quantitative electron microscopy. The Golgi stacks in these GRASP-deficient HeLa cells were normal both in morphology and in anterograde cargo transport. This suggests the simple hypothesis that the total amount of adhesive energy gluing cisternae dictates Golgi cisternal stacking, irrespective of which molecules mediate the adhesive process. In support of this hypothesis, we show that adding artificial adhesive energy between cisternae and mitochondria by dimerizing rapamycin-binding domain and FK506-binding protein domains that are attached to cisternal adhesive proteins allows mitochondria to invade the stack and even replace Golgi cisternae within a few hours. These results indicate that although Golgi stacking is a highly complicated process involving a large number of adhesive and regulatory proteins, the overriding principle of a Golgi stack assembly is likely to be quite simple. From this simplified perspective, we propose a model, based on cisternal adhesion and cisternal maturation as the two core principles, illustrating how the most ancient form of Golgi stacking might have occurred using only weak cisternal adhesive processes because of the differential between the rate of influx and outflux of membrane transport through the Golgi.
Keywords: GRASPs; tethers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
GRASP55 and GRASP65 play complementary and essential roles in Golgi cisternal stacking.J Cell Biol. 2010 Jan 25;188(2):237-51. doi: 10.1083/jcb.200907132. Epub 2010 Jan 18. J Cell Biol. 2010. PMID: 20083603 Free PMC article.
-
GRASP55, a second mammalian GRASP protein involved in the stacking of Golgi cisternae in a cell-free system.EMBO J. 1999 Sep 15;18(18):4949-60. doi: 10.1093/emboj/18.18.4949. EMBO J. 1999. PMID: 10487747 Free PMC article.
-
A role for the vesicle tethering protein, p115, in the post-mitotic stacking of reassembling Golgi cisternae in a cell-free system.J Cell Biol. 1999 Jul 12;146(1):57-70. doi: 10.1083/jcb.146.1.57. J Cell Biol. 1999. PMID: 10402460 Free PMC article.
-
The multiple facets of the Golgi reassembly stacking proteins.Biochem J. 2011 Feb 1;433(3):423-33. doi: 10.1042/BJ20101540. Biochem J. 2011. PMID: 21235525 Review.
-
A cisternal maturation mechanism can explain the asymmetry of the Golgi stack.FEBS Lett. 1997 Sep 8;414(2):177-81. doi: 10.1016/s0014-5793(97)00984-8. FEBS Lett. 1997. PMID: 9315681 Review.
Cited by
-
DjA1 maintains Golgi integrity via interaction with GRASP65.Mol Biol Cell. 2019 Feb 15;30(4):478-490. doi: 10.1091/mbc.E18-10-0613. Epub 2018 Dec 19. Mol Biol Cell. 2019. PMID: 30566031 Free PMC article.
-
Acylation - A New Means to Control Traffic Through the Golgi.Front Cell Dev Biol. 2019 Jun 12;7:109. doi: 10.3389/fcell.2019.00109. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31245373 Free PMC article. Review.
-
Golgi defect as a major contributor to lysosomal dysfunction.Front Cell Dev Biol. 2024 Apr 24;12:1386149. doi: 10.3389/fcell.2024.1386149. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38721528 Free PMC article. Review.
-
Non-canonical features of the Golgi apparatus in bipolar epithelial neural stem cells.Sci Rep. 2016 Feb 16;6:21206. doi: 10.1038/srep21206. Sci Rep. 2016. PMID: 26879757 Free PMC article.
-
Aging and MPTP Sensitivity Depend on Molecular and Ultrastructural Signatures of Astroglia and Microglia in Mice Substantia Nigra.Cell Mol Neurobiol. 2025 Jan 20;45(1):13. doi: 10.1007/s10571-024-01528-8. Cell Mol Neurobiol. 2025. PMID: 39833644 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

