Doxorubicin-induced carbonylation and degradation of cardiac myosin binding protein C promote cardiotoxicity
- PMID: 24449919
- PMCID: PMC3918758
- DOI: 10.1073/pnas.1321783111
Doxorubicin-induced carbonylation and degradation of cardiac myosin binding protein C promote cardiotoxicity
Abstract
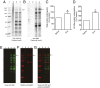
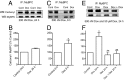
Dose-dependent oxidative stress by the anthracycline doxorubicin (Dox) and other chemotherapeutic agents causes irreversible cardiac damage, restricting their clinical effectiveness. We hypothesized that the resultant protein oxidation could be monitored and correlated with physiological functional impairment. We focused on protein carbonylation as an indicator of severe oxidative damage because it is irreversible and results in proteasomal degradation. We identified and investigated a specific high-molecular weight cardiac protein that showed a significant increase in carbonylation under Dox-induced cardiotoxic conditions in a spontaneously hypertensive rat model. We confirmed carbonylation and degradation of this protein under oxidative stress and prevention of such effect in the presence of the iron chelator dexrazoxane. Using MS, the Dox-induced carbonylated protein was identified as the 140-kDa cardiac myosin binding protein C (MyBPC). We confirmed the carbonylation and degradation of MyBPC using HL-1 cardiomyocytes and a purified recombinant untagged cardiac MyBPC under metal-catalyzed oxidative stress conditions. The carbonylation and degradation of MyBPC were time- and drug concentration-dependent. We demonstrated that carbonylated MyBPC undergoes proteasome-mediated degradation under Dox-induced oxidative stress. Cosedimentation, immunoprecipitation, and actin binding assays were used to study the functional consequences of carbonylated MyBPC. Carbonylation of MyBPC showed significant functional impairment associated with its actin binding properties. The dissociation constant of carbonylated recombinant MyBPC for actin was 7.35 ± 1.9 μM compared with 2.7 ± 0.6 μM for native MyBPC. Overall, our findings indicate that MyBPC carbonylation serves as a critical determinant of cardiotoxicity and could serve as a mechanistic indicator for Dox-induced cardiotoxicity.
Keywords: ROS; cancer; cardioprotection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Mito-tempol and dexrazoxane exhibit cardioprotective and chemotherapeutic effects through specific protein oxidation and autophagy in a syngeneic breast tumor preclinical model.PLoS One. 2013 Aug 5;8(8):e70575. doi: 10.1371/journal.pone.0070575. Print 2013. PLoS One. 2013. PMID: 23940596 Free PMC article.
-
Doxorubicin-induced cardiotoxicity is suppressed by estrous-staged treatment and exogenous 17β-estradiol in female tumor-bearing spontaneously hypertensive rats.Biol Sex Differ. 2018 Jun 15;9(1):25. doi: 10.1186/s13293-018-0183-9. Biol Sex Differ. 2018. PMID: 29907135 Free PMC article.
-
Resolvin E1 protects against doxorubicin-induced cardiotoxicity by inhibiting oxidative stress, autophagy and apoptosis by targeting AKT/mTOR signaling.Biochem Pharmacol. 2020 Oct;180:114188. doi: 10.1016/j.bcp.2020.114188. Epub 2020 Aug 1. Biochem Pharmacol. 2020. PMID: 32750329
-
Oxidative stress injury in doxorubicin-induced cardiotoxicity.Toxicol Lett. 2019 Jun 1;307:41-48. doi: 10.1016/j.toxlet.2019.02.013. Epub 2019 Feb 25. Toxicol Lett. 2019. PMID: 30817977 Review.
-
Protein carbonylation and metal-catalyzed protein oxidation in a cellular perspective.J Proteomics. 2011 Oct 19;74(11):2228-42. doi: 10.1016/j.jprot.2011.05.004. Epub 2011 May 11. J Proteomics. 2011. PMID: 21601020 Review.
Cited by
-
Role of Inflammation and Redox Status on Doxorubicin-Induced Cardiotoxicity in Infant and Adult CD-1 Male Mice.Biomolecules. 2021 Nov 19;11(11):1725. doi: 10.3390/biom11111725. Biomolecules. 2021. PMID: 34827723 Free PMC article.
-
Protein Carbonylation and Lipid Peroxidation in Hematological Malignancies.Antioxidants (Basel). 2020 Dec 1;9(12):1212. doi: 10.3390/antiox9121212. Antioxidants (Basel). 2020. PMID: 33271863 Free PMC article. Review.
-
Advances in the Study of Protein Deamidation: Unveiling Its Influence on Aging, Disease Progression, Forensics and Therapeutic Efficacy.Proteomes. 2025 Jun 5;13(2):24. doi: 10.3390/proteomes13020024. Proteomes. 2025. PMID: 40559997 Free PMC article. Review.
-
The ET-1-mediated carbonylation and degradation of ANXA1 induce inflammatory phenotype and proliferation of pulmonary artery smooth muscle cells in HPS.PLoS One. 2017 Apr 17;12(4):e0175443. doi: 10.1371/journal.pone.0175443. eCollection 2017. PLoS One. 2017. PMID: 28414743 Free PMC article.
-
Involvement of oxidative modification of proteins related to ATP synthesis in the left ventricles of hamsters with cardiomyopathy.Sci Rep. 2017 Aug 23;7(1):9243. doi: 10.1038/s41598-017-08546-1. Sci Rep. 2017. PMID: 28835655 Free PMC article.
References
-
- Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 2010;38(1):96–109. - PubMed
-
- Lipshultz SE, et al. American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Basic Cardiovascular Sciences, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Radiolo Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association. Circulation. 2013;128(17):1927–1995. - PubMed
-
- Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991;266(12):1672–1677. - PubMed
-
- Xu XX, Persson HL, Richardson DR. Molecular pharmacology of the interaction of anthracyclines with iron. Mol Pharmacol. 2005;68(2):261–271. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

