Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants
- PMID: 24450576
- PMCID: PMC3971624
- DOI: 10.1186/2049-2618-1-13
Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants
Abstract
Background: Necrotizing enterocolitis (NEC) is a devastating intestinal disease that afflicts 10% of extremely preterm infants. The contribution of early intestinal colonization to NEC onset is not understood, and predictive biomarkers to guide prevention are lacking. We analyzed banked stool and urine samples collected prior to disease onset from infants <29 weeks gestational age, including 11 infants who developed NEC and 21 matched controls who survived free of NEC. Stool bacterial communities were profiled by 16S rRNA gene sequencing. Urinary metabolomic profiles were assessed by NMR.
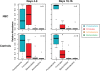
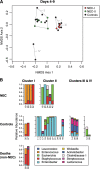
Results: During postnatal days 4 to 9, samples from infants who later developed NEC tended towards lower alpha diversity (Chao1 index, P = 0.086) and lacked Propionibacterium (P = 0.009) compared to controls. Furthermore, NEC was preceded by distinct forms of dysbiosis. During days 4 to 9, samples from four NEC cases were dominated by members of the Firmicutes (median relative abundance >99% versus <17% in the remaining NEC and controls, P < 0.001). During postnatal days 10 to 16, samples from the remaining NEC cases were dominated by Proteobacteria, specifically Enterobacteriaceae (median relative abundance >99% versus 38% in the other NEC cases and 84% in controls, P = 0.01). NEC preceded by Firmicutes dysbiosis occurred earlier (onset, days 7 to 21) than NEC preceded by Proteobacteria dysbiosis (onset, days 19 to 39). All NEC cases lacked Propionibacterium and were preceded by either Firmicutes (≥98% relative abundance, days 4 to 9) or Proteobacteria (≥90% relative abundance, days 10 to 16) dysbiosis, while only 25% of controls had this phenotype (predictive value 88%, P = 0.001). Analysis of days 4 to 9 urine samples found no metabolites associated with all NEC cases, but alanine was positively associated with NEC cases that were preceded by Firmicutes dysbiosis (P < 0.001) and histidine was inversely associated with NEC cases preceded by Proteobacteria dysbiosis (P = 0.013). A high urinary alanine:histidine ratio was associated with microbial characteristics (P < 0.001) and provided good prediction of overall NEC (predictive value 78%, P = 0.007).
Conclusions: Early dysbiosis is strongly involved in the pathobiology of NEC. These striking findings require validation in larger studies but indicate that early microbial and metabolomic signatures may provide highly predictive biomarkers of NEC.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

