Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease
- PMID: 24450891
- PMCID: PMC4159618
- DOI: 10.1056/NEJMoa1304839
Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease
Abstract
Background: Bapineuzumab, a humanized anti-amyloid-beta monoclonal antibody, is in clinical development for the treatment of Alzheimer's disease.
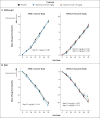
Methods: We conducted two double-blind, randomized, placebo-controlled, phase 3 trials involving patients with mild-to-moderate Alzheimer's disease--one involving 1121 carriers of the apolipoprotein E (APOE) ε4 allele and the other involving 1331 noncarriers. Bapineuzumab or placebo, with doses varying by study, was administered by intravenous infusion every 13 weeks for 78 weeks. The primary outcome measures were scores on the 11-item cognitive subscale of the Alzheimer's Disease Assessment Scale (ADAS-cog11, with scores ranging from 0 to 70 and higher scores indicating greater impairment) and the Disability Assessment for Dementia (DAD, with scores ranging from 0 to 100 and higher scores indicating less impairment). A total of 1090 carriers and 1114 noncarriers were included in the efficacy analysis. Secondary outcome measures included findings on positron-emission tomographic amyloid imaging with the use of Pittsburgh compound B (PIB-PET) and cerebrospinal fluid phosphorylated tau (phospho-tau) concentrations.
Results: There were no significant between-group differences in the primary outcomes. At week 78, the between-group differences in the change from baseline in the ADAS-cog11 and DAD scores (bapineuzumab group minus placebo group) were -0.2 (P=0.80) and -1.2 (P=0.34), respectively, in the carrier study; the corresponding differences in the noncarrier study were -0.3 (P=0.64) and 2.8 (P=0.07) with the 0.5-mg-per-kilogram dose of bapineuzumab and 0.4 (P=0.62) and 0.9 (P=0.55) with the 1.0-mg-per-kilogram dose. The major safety finding was amyloid-related imaging abnormalities with edema among patients receiving bapineuzumab, which increased with bapineuzumab dose and APOE ε4 allele number and which led to discontinuation of the 2.0-mg-per-kilogram dose. Between-group differences were observed with respect to PIB-PET and cerebrospinal fluid phospho-tau concentrations in APOE ε4 allele carriers but not in noncarriers.
Conclusions: Bapineuzumab did not improve clinical outcomes in patients with Alzheimer's disease, despite treatment differences in biomarkers observed in APOE ε4 carriers. (Funded by Janssen Alzheimer Immunotherapy and Pfizer; Bapineuzumab 301 and 302 ClinicalTrials.gov numbers, NCT00575055 and NCT00574132, and EudraCT number, 2009-012748-17.).
Figures

Comment in
-
Antiamyloid therapy for Alzheimer's disease--are we on the right road?N Engl J Med. 2014 Jan 23;370(4):377-8. doi: 10.1056/NEJMe1313943. N Engl J Med. 2014. PMID: 24450897 No abstract available.
-
[Do antibodies provide advantages for the therapy of Alzheimer's disease? Solanezumab: the jury is still out].Dtsch Med Wochenschr. 2014 Mar;139(10):468. doi: 10.1055/s-0033-1353892. Epub 2014 Feb 25. Dtsch Med Wochenschr. 2014. PMID: 24570189 German. No abstract available.
-
Phase 3 trials of solanezumab and bapineuzumab for Alzheimer's disease.N Engl J Med. 2014 Apr 10;370(15):1459. doi: 10.1056/NEJMc1402193. N Engl J Med. 2014. PMID: 24716688 No abstract available.
-
Phase 3 trials of solanezumab and bapineuzumab for Alzheimer's disease.N Engl J Med. 2014 Apr 10;370(15):1460. doi: 10.1056/NEJMc1402193. N Engl J Med. 2014. PMID: 24724181 No abstract available.
References
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. [Erratum, Science 2002;297:2209.] - PubMed
-
- Bard F, Cannon C, Barbour R, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
