NAADP and the two-pore channel protein 1 participate in the acrosome reaction in mammalian spermatozoa
- PMID: 24451262
- PMCID: PMC3952862
- DOI: 10.1091/mbc.E13-09-0523
NAADP and the two-pore channel protein 1 participate in the acrosome reaction in mammalian spermatozoa
Abstract
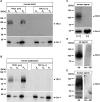
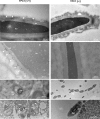
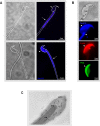
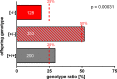
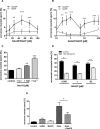
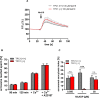
The functional relationship between the formation of hundreds of fusion pores during the acrosome reaction in spermatozoa and the mobilization of calcium from the acrosome has been determined only partially. Hence, the second messenger NAADP, promoting efflux of calcium from lysosome-like compartments and one of its potential molecular targets, the two-pore channel 1 (TPC1), were analyzed for its involvement in triggering the acrosome reaction using a TPCN1 gene-deficient mouse strain. The present study documents that TPC1 and NAADP-binding sites showed a colocalization at the acrosomal region and that treatment of spermatozoa with NAADP resulted in a loss of the acrosomal vesicle that showed typical properties described for TPCs: Registered responses were not detectable for its chemical analogue NADP and were blocked by the NAADP antagonist trans-Ned-19. In addition, two narrow bell-shaped dose-response curves were identified with maxima in either the nanomolar or low micromolar NAADP concentration range, where TPC1 was found to be responsible for activating the low affinity pathway. Our finding that two convergent NAADP-dependent pathways are operative in driving acrosomal exocytosis supports the concept that both NAADP-gated cascades match local NAADP concentrations with the efflux of acrosomal calcium, thereby ensuring complete fusion of the large acrosomal vesicle.
Figures
Similar articles
-
The calcium-mobilizing messenger nicotinic acid adenine dinucleotide phosphate participates in sperm activation by mediating the acrosome reaction.J Biol Chem. 2010 Jun 11;285(24):18262-9. doi: 10.1074/jbc.M109.087858. Epub 2010 Apr 16. J Biol Chem. 2010. PMID: 20400502 Free PMC article.
-
Expression of Ca²⁺-permeable two-pore channels rescues NAADP signalling in TPC-deficient cells.EMBO J. 2015 Jul 2;34(13):1743-58. doi: 10.15252/embj.201490009. Epub 2015 Apr 14. EMBO J. 2015. PMID: 25872774 Free PMC article.
-
Inhibition of NAADP signalling on reperfusion protects the heart by preventing lethal calcium oscillations via two-pore channel 1 and opening of the mitochondrial permeability transition pore.Cardiovasc Res. 2015 Dec 1;108(3):357-66. doi: 10.1093/cvr/cvv226. Epub 2015 Sep 22. Cardiovasc Res. 2015. PMID: 26395965 Free PMC article.
-
Calcium signaling via two-pore channels: local or global, that is the question.Am J Physiol Cell Physiol. 2010 Mar;298(3):C430-41. doi: 10.1152/ajpcell.00475.2009. Epub 2009 Dec 16. Am J Physiol Cell Physiol. 2010. PMID: 20018950 Free PMC article. Review.
-
Activation of endo-lysosomal two-pore channels by NAADP and PI(3,5)P2. Five things to know.Cell Calcium. 2022 May;103:102543. doi: 10.1016/j.ceca.2022.102543. Epub 2022 Jan 25. Cell Calcium. 2022. PMID: 35123238 Free PMC article. Review.
Cited by
-
TPC1 deficiency or blockade augments systemic anaphylaxis and mast cell activity.Proc Natl Acad Sci U S A. 2020 Jul 28;117(30):18068-18078. doi: 10.1073/pnas.1920122117. Epub 2020 Jul 13. Proc Natl Acad Sci U S A. 2020. PMID: 32661165 Free PMC article.
-
Targeting Two-Pore Channels: Current Progress and Future Challenges.Trends Pharmacol Sci. 2020 Aug;41(8):582-594. doi: 10.1016/j.tips.2020.06.002. Trends Pharmacol Sci. 2020. PMID: 32679067 Free PMC article. Review.
-
Two-pore Channels (TPC2s) and Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP) at Lysosomal-Sarcoplasmic Reticular Junctions Contribute to Acute and Chronic β-Adrenoceptor Signaling in the Heart.J Biol Chem. 2015 Dec 11;290(50):30087-98. doi: 10.1074/jbc.M115.684076. Epub 2015 Oct 5. J Biol Chem. 2015. PMID: 26438825 Free PMC article.
-
TPC1 has two variant isoforms, and their removal has different effects on endo-lysosomal functions compared to loss of TPC2.Mol Cell Biol. 2014 Nov;34(21):3981-92. doi: 10.1128/MCB.00113-14. Epub 2014 Aug 18. Mol Cell Biol. 2014. PMID: 25135478 Free PMC article.
-
Metabolic alterations derived from absence of Two-Pore Channel 1 at cardiac level.J Biosci. 2016 Dec;41(4):643-658. doi: 10.1007/s12038-016-9647-4. J Biosci. 2016. PMID: 27966485
References
-
- Aarhus R, Dickey DM, Graeff RM, Gee KR, Walseth TF, Lee HC. Activation and inactivation of Ca2+ release by NAADP+ J Biol Chem. 1996;271:8513–8516. - PubMed
-
- Abou-Haila A, Tulsiani DR. Signal transduction pathways that regulate sperm capacitation and the acrosome reaction. Arch Biochem Biophys. 2009;485:72–81. - PubMed
-
- Ackermann F, Zitranski N, Borth H, Buech T, Gudermann T, Boekhoff I. CaMKIIalpha interacts with multi-PDZ domain protein MUPP1 in spermatozoa and prevents spontaneous acrosomal exocytosis. J Cell Sci. 2009;122:4547–4557. - PubMed
-
- Ackermann F, Zitranski N, Heydecke D, Wilhelm B, Gudermann T, Boekhoff I. The multi-PDZ domain protein MUPP1 as a lipid raft-associated scaffolding protein controlling the acrosome reaction in mammalian spermatozoa. J Cell Physiol. 2008;214:757–768. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

