Shear stress-induced redistribution of vascular endothelial-protein-tyrosine phosphatase (VE-PTP) in endothelial cells and its role in cell elongation
- PMID: 24451369
- PMCID: PMC3945311
- DOI: 10.1074/jbc.M113.529503
Shear stress-induced redistribution of vascular endothelial-protein-tyrosine phosphatase (VE-PTP) in endothelial cells and its role in cell elongation
Abstract
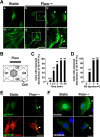
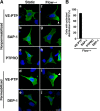
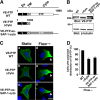
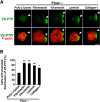
Vascular endothelial cells (ECs) are continuously exposed to shear stress (SS) generated by blood flow. Such stress plays a key role in regulation of various aspects of EC function including cell proliferation and motility as well as changes in cell morphology. Vascular endothelial-protein-tyrosine phosphatase (VE-PTP) is an R3-subtype PTP that possesses multiple fibronectin type III-like domains in its extracellular region and is expressed specifically in ECs. The role of VE-PTP in EC responses to SS has remained unknown, however. Here we show that VE-PTP is diffusely localized in ECs maintained under static culture conditions, whereas it undergoes rapid accumulation at the downstream edge of the cells relative to the direction of flow in response to SS. This redistribution of VE-PTP triggered by SS was found to require its extracellular and transmembrane regions and was promoted by integrin engagement of extracellular matrix ligands. Inhibition of actin polymerization or of Cdc42, Rab5, or Arf6 activities attenuated the SS-induced redistribution of VE-PTP. VE-PTP also underwent endocytosis in the static and SS conditions. SS induced the polarized distribution of internalized VE-PTP. Such an effect was promoted by integrin engagement of fibronectin but prevented by inhibition of Cdc42 activity or of actin polymerization. In addition, depletion of VE-PTP by RNA interference in human umbilical vein ECs blocked cell elongation in the direction of flow induced by SS. Our results suggest that the polarized redistribution of VE-PTP in response to SS plays an important role in the regulation of EC function by blood flow.
Keywords: Endothelial Cell; Membrane Proteins; Protein-tyrosine Phosphatase (Tyrosine Phosphatase); Shear Stress; Small GTPases.
Figures
Similar articles
-
Promotion of cell spreading and migration by vascular endothelial-protein tyrosine phosphatase (VE-PTP) in cooperation with integrins.J Cell Physiol. 2010 Jul;224(1):195-204. doi: 10.1002/jcp.22122. J Cell Physiol. 2010. PMID: 20301196
-
VE-PTP inhibition stabilizes endothelial junctions by activating FGD5.EMBO Rep. 2019 Jul;20(7):e47046. doi: 10.15252/embr.201847046. Epub 2019 May 14. EMBO Rep. 2019. PMID: 31267715 Free PMC article.
-
VE-PTP inhibition elicits eNOS phosphorylation to blunt endothelial dysfunction and hypertension in diabetes.Cardiovasc Res. 2021 May 25;117(6):1546-1556. doi: 10.1093/cvr/cvaa213. Cardiovasc Res. 2021. PMID: 32653904 Clinical Trial.
-
Vascular Endothelial Protein Tyrosine Phosphatase Regulates Endothelial Function.Physiology (Bethesda). 2021 Mar 1;36(2):84-93. doi: 10.1152/physiol.00026.2020. Physiology (Bethesda). 2021. PMID: 33595386 Review.
-
Expression, localization, and biological function of the R3 subtype of receptor-type protein tyrosine phosphatases in mammals.Cell Signal. 2010 Dec;22(12):1811-7. doi: 10.1016/j.cellsig.2010.07.001. Epub 2010 Jul 12. Cell Signal. 2010. PMID: 20633639 Review.
Cited by
-
Actin cytoskeleton in angiogenesis.Biol Open. 2022 Dec 15;11(12):bio058899. doi: 10.1242/bio.058899. Epub 2022 Nov 29. Biol Open. 2022. PMID: 36444960 Free PMC article. Review.
-
Hemodynamic shear stress via ROS modulates PCSK9 expression in human vascular endothelial and smooth muscle cells and along the mouse aorta.Antioxid Redox Signal. 2015 Mar 20;22(9):760-71. doi: 10.1089/ars.2014.6054. Epub 2015 Jan 8. Antioxid Redox Signal. 2015. PMID: 25490141 Free PMC article.
-
VE-PTP controls a fluid shear stress set point that governs cell morphological responses through Tie-2.Front Cell Dev Biol. 2025 Jul 4;13:1603517. doi: 10.3389/fcell.2025.1603517. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40688354 Free PMC article.
-
Shear stress control of vascular leaks and atheromas through Tie2 activation by VE-PTP sequestration.EMBO Mol Med. 2023 Apr 11;15(4):e16128. doi: 10.15252/emmm.202216128. Epub 2023 Feb 6. EMBO Mol Med. 2023. PMID: 36740996 Free PMC article.
-
To Fuse or Not to Fuse.Arterioscler Thromb Vasc Biol. 2018 Sep;38(9):1959-1960. doi: 10.1161/ATVBAHA.118.311463. Arterioscler Thromb Vasc Biol. 2018. PMID: 30354263 Free PMC article. No abstract available.
References
-
- Davies P. F., Mundel T., Barbee K. A. (1995) A mechanism for heterogeneous endothelial responses to flow in vivo and in vitro. J. biomech. 28, 1553–1560 - PubMed
-
- Ando J., Yamamoto K. (2009) Vascular mechanobiology. Endothelial cell responses to fluid shear stress. Circ. J. 73, 1983–1992 - PubMed
-
- Li Y. S., Haga J. H., Chien S. (2005) Molecular basis of the effects of shear stress on vascular endothelial cells. J. Biomech. 38, 1949–1971 - PubMed
-
- Tai L. K., Okuda M., Abe J., Yan C., Berk B. C. (2002) Fluid shear stress activates proline-rich tyrosine kinase via reactive oxygen species-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 22, 1790–1796 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

