Endocytosis and vacuolar degradation of the yeast cell surface glucose sensors Rgt2 and Snf3
- PMID: 24451370
- PMCID: PMC3945383
- DOI: 10.1074/jbc.M113.539411
Endocytosis and vacuolar degradation of the yeast cell surface glucose sensors Rgt2 and Snf3
Retraction in
-
Endocytosis and vacuolar degradation of the yeast cell surface glucose sensors Rgt2 and Snf3.J Biol Chem. 2016 Jul 15;291(29):14913. doi: 10.1074/jbc.A113.539411. J Biol Chem. 2016. PMID: 27422977 Free PMC article. No abstract available.
Abstract
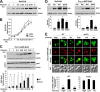
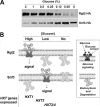
Sensing and signaling the presence of extracellular glucose is crucial for the yeast Saccharomyces cerevisiae because of its fermentative metabolism, characterized by high glucose flux through glycolysis. The yeast senses glucose through the cell surface glucose sensors Rgt2 and Snf3, which serve as glucose receptors that generate the signal for induction of genes involved in glucose uptake and metabolism. Rgt2 and Snf3 detect high and low glucose concentrations, respectively, perhaps because of their different affinities for glucose. Here, we provide evidence that cell surface levels of glucose sensors are regulated by ubiquitination and degradation. The glucose sensors are removed from the plasma membrane through endocytosis and targeted to the vacuole for degradation upon glucose depletion. The turnover of the glucose sensors is inhibited in endocytosis defective mutants, and the sensor proteins with a mutation at their putative ubiquitin-acceptor lysine residues are resistant to degradation. Of note, the low affinity glucose sensor Rgt2 remains stable only in high glucose grown cells, and the high affinity glucose sensor Snf3 is stable only in cells grown in low glucose. In addition, constitutively active, signaling forms of glucose sensors do not undergo endocytosis, whereas signaling defective sensors are constitutively targeted for degradation, suggesting that the stability of the glucose sensors may be associated with their ability to sense glucose. Therefore, our findings demonstrate that the amount of glucose available dictates the cell surface levels of the glucose sensors and that the regulation of glucose sensors by glucose concentration may enable yeast cells to maintain glucose sensing activity at the cell surface over a wide range of glucose concentrations.
Keywords: Cell Biology; Gene Expression; Glucose; Receptors; Yeast.
Figures




Similar articles
-
The glucose metabolite methylglyoxal inhibits expression of the glucose transporter genes by inactivating the cell surface glucose sensors Rgt2 and Snf3 in yeast.Mol Biol Cell. 2016 Mar 1;27(5):862-71. doi: 10.1091/mbc.E15-11-0789. Epub 2016 Jan 13. Mol Biol Cell. 2016. PMID: 26764094 Free PMC article.
-
Glucose regulation of the paralogous glucose sensing receptors Rgt2 and Snf3 of the yeast Saccharomyces cerevisiae.Biochim Biophys Acta Gen Subj. 2021 Jun;1865(6):129881. doi: 10.1016/j.bbagen.2021.129881. Epub 2021 Feb 20. Biochim Biophys Acta Gen Subj. 2021. PMID: 33617932
-
Casein kinases are required for the stability of the glucose-sensing receptor Rgt2 in yeast.Sci Rep. 2022 Jan 31;12(1):1598. doi: 10.1038/s41598-022-05569-1. Sci Rep. 2022. PMID: 35102180 Free PMC article.
-
How do yeast cells sense glucose?Bioessays. 1998 Dec;20(12):972-6. doi: 10.1002/(SICI)1521-1878(199812)20:12<972::AID-BIES2>3.0.CO;2-M. Bioessays. 1998. PMID: 10048296 Review.
-
Glucose repression in Saccharomyces cerevisiae.FEMS Yeast Res. 2015 Sep;15(6):fov068. doi: 10.1093/femsyr/fov068. Epub 2015 Jul 22. FEMS Yeast Res. 2015. PMID: 26205245 Free PMC article. Review.
Cited by
-
Multiple Transceptors for Macro- and Micro-Nutrients Control Diverse Cellular Properties Through the PKA Pathway in Yeast: A Paradigm for the Rapidly Expanding World of Eukaryotic Nutrient Transceptors Up to Those in Human Cells.Front Pharmacol. 2018 Mar 13;9:191. doi: 10.3389/fphar.2018.00191. eCollection 2018. Front Pharmacol. 2018. PMID: 29662449 Free PMC article. Review.
-
Genetic Evidence for the Role of the Vacuole in Supplying Secretory Organelles with Ca2+ in Hansenula polymorpha.PLoS One. 2015 Dec 30;10(12):e0145915. doi: 10.1371/journal.pone.0145915. eCollection 2015. PLoS One. 2015. PMID: 26717478 Free PMC article.
-
The glucose metabolite methylglyoxal inhibits expression of the glucose transporter genes by inactivating the cell surface glucose sensors Rgt2 and Snf3 in yeast.Mol Biol Cell. 2016 Mar 1;27(5):862-71. doi: 10.1091/mbc.E15-11-0789. Epub 2016 Jan 13. Mol Biol Cell. 2016. PMID: 26764094 Free PMC article.
-
Glycolysis controls plasma membrane glucose sensors to promote glucose signaling in yeasts.Mol Cell Biol. 2015 Feb;35(4):747-57. doi: 10.1128/MCB.00515-14. Epub 2014 Dec 15. Mol Cell Biol. 2015. PMID: 25512610 Free PMC article.
-
A novel role for yeast casein kinases in glucose sensing and signaling.Mol Biol Cell. 2016 Nov 1;27(21):3369-3375. doi: 10.1091/mbc.E16-05-0342. Epub 2016 Sep 14. Mol Biol Cell. 2016. PMID: 27630263 Free PMC article.
References
-
- Rolland F., Wanke V., Cauwenberg L., Ma P., Boles E., Vanoni M., de Winde J. H., Thevelein J. M., Winderickx J. (2001) The role of hexose transport and phosphorylation in cAMP signalling in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 1, 33–45 - PubMed
-
- Towle H. C. (2005) Glucose as a regulator of eukaryotic gene transcription. Trends Endocrinol. Metab. 16, 489–494 - PubMed
-
- Holsbeeks I., Lagatie O., Van Nuland A., Van de Velde S., Thevelein J. M. (2004) The eukaryotic plasma membrane as a nutrient-sensing device. Trends Biochem. Sci. 29, 556–564 - PubMed
-
- Johnston M., Kim J. H. (2005) Glucose as a hormone. Receptor-mediated glucose sensing in the yeast Saccharomyces cerevisiae. Biochem. Soc. Trans. 33, 247–252 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

