Vaccinia viral protein A27 is anchored to the viral membrane via a cooperative interaction with viral membrane protein A17
- PMID: 24451374
- PMCID: PMC3945326
- DOI: 10.1074/jbc.M114.547372
Vaccinia viral protein A27 is anchored to the viral membrane via a cooperative interaction with viral membrane protein A17
Abstract
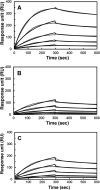
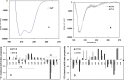
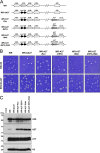
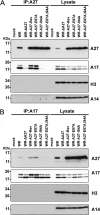
The vaccinia viral protein A27 in mature viruses specifically interacts with heparan sulfate for cell surface attachment. In addition, A27 associates with the viral membrane protein A17 to anchor to the viral membrane; however, the specific interaction between A27 and A17 remains largely unclear. To uncover the active binding sites and the underlying binding mechanism, we expressed and purified the N-terminal (18-50 residues) and C-terminal (162-203 residues) fragments of A17, which are denoted A17-N and A17-C. Through surface plasmon resonance, the binding affinity of A27/A17-N (KA = 3.40 × 10(8) m(-1)) was determined to be approximately 3 orders of magnitude stronger than that of A27/A17-C (KA = 3.40 × 10(5) m(-1)), indicating that A27 prefers to interact with A17-N rather than A17-C. Despite the disordered nature of A17-N, the A27-A17 interaction is mediated by a specific and cooperative binding mechanism that includes two active binding sites, namely (32)SFMPK(36) (denoted as F1 binding) and (20)LDKDLFTEEQ(29) (F2). Further analysis showed that F1 has stronger binding affinity and is more resistant to acidic conditions than is F2. Furthermore, A27 mutant proteins that retained partial activity to interact with the F1 and F2 sites of the A17 protein were packaged into mature virus particles at a reduced level, demonstrating that the F1/F2 interaction plays a critical role in vivo. Using these results in combination with site-directed mutagenesis data, we established a computer model to explain the specific A27-A17 binding mechanism.
Keywords: Pox Viruses; Protein Structure; Protein-Protein Interactions; Viral Protein; Virus Assembly.
Figures




Similar articles
-
A turn-like structure "KKPE" segment mediates the specific binding of viral protein A27 to heparin and heparan sulfate on cell surfaces.J Biol Chem. 2009 Dec 25;284(52):36535-36546. doi: 10.1074/jbc.M109.037267. Epub 2009 Oct 26. J Biol Chem. 2009. PMID: 19858217 Free PMC article.
-
Membrane cell fusion activity of the vaccinia virus A17-A27 protein complex.Cell Microbiol. 2008 Jan;10(1):149-64. doi: 10.1111/j.1462-5822.2007.01026.x. Epub 2007 Aug 15. Cell Microbiol. 2008. PMID: 17708756
-
Crystal structure of vaccinia viral A27 protein reveals a novel structure critical for its function and complex formation with A26 protein.PLoS Pathog. 2013;9(8):e1003563. doi: 10.1371/journal.ppat.1003563. Epub 2013 Aug 22. PLoS Pathog. 2013. PMID: 23990784 Free PMC article.
-
Disulfide bond formation at the C termini of vaccinia virus A26 and A27 proteins does not require viral redox enzymes and suppresses glycosaminoglycan-mediated cell fusion.J Virol. 2009 Jul;83(13):6464-76. doi: 10.1128/JVI.02295-08. Epub 2009 Apr 15. J Virol. 2009. PMID: 19369327 Free PMC article.
-
Proteins that bind high-mannose sugars of the HIV envelope.Prog Biophys Mol Biol. 2005 Jun;88(2):233-82. doi: 10.1016/j.pbiomolbio.2004.05.001. Prog Biophys Mol Biol. 2005. PMID: 15572157 Review.
Cited by
-
The vaccinia chondroitin sulfate binding protein drives host membrane curvature to facilitate fusion.EMBO Rep. 2024 Mar;25(3):1310-1325. doi: 10.1038/s44319-023-00040-2. Epub 2024 Feb 6. EMBO Rep. 2024. PMID: 38321165 Free PMC article.
-
Inactivation of Genes by Frameshift Mutations Provides Rapid Adaptation of an Attenuated Vaccinia Virus.J Virol. 2020 Aug 31;94(18):e01053-20. doi: 10.1128/JVI.01053-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32669330 Free PMC article.
-
Functional epitopes and neutralizing antibodies of vaccinia virus.Front Microbiol. 2023 Oct 25;14:1255935. doi: 10.3389/fmicb.2023.1255935. eCollection 2023. Front Microbiol. 2023. PMID: 37954238 Free PMC article. Review.
-
The Vaccinia virion: Filling the gap between atomic and ultrastructure.PLoS Pathog. 2019 Jan 7;15(1):e1007508. doi: 10.1371/journal.ppat.1007508. eCollection 2019 Jan. PLoS Pathog. 2019. PMID: 30615658 Free PMC article.
-
Structural basis for the inhibition of poxvirus assembly by the antibiotic rifampicin.Proc Natl Acad Sci U S A. 2018 Aug 14;115(33):8424-8429. doi: 10.1073/pnas.1810398115. Epub 2018 Aug 1. Proc Natl Acad Sci U S A. 2018. PMID: 30068608 Free PMC article.
References
-
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. (1990) The complete DNA sequence of vaccinia virus. Virology 179, 247–266 - PubMed
-
- Moss B. (1996) in Fields Virology (Fields B. N., Knipe D. M., Howley P. M., eds) 3rd Ed., pp. 2637–2671, Lippincott-Raven Publishers, Philadelphia
-
- Resch W., Hixson K. K., Moore R. J., Lipton M. S., Moss B. (2007) Protein composition of the vaccinia virus mature virion. Virology 358, 233–247 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

