In situ dimerization of multiple wild type and mutant zinc transporters in live cells using bimolecular fluorescence complementation
- PMID: 24451381
- PMCID: PMC3953246
- DOI: 10.1074/jbc.M113.533786
In situ dimerization of multiple wild type and mutant zinc transporters in live cells using bimolecular fluorescence complementation
Abstract
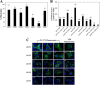
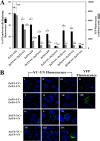
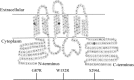
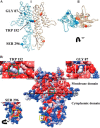
Zinc transporters (ZnTs) facilitate zinc efflux and zinc compartmentalization, thereby playing a key role in multiple physiological processes and pathological disorders, presumed to be modulated by transporter dimerization. We recently proposed that ZnT2 homodimerization is the underlying basis for the dominant negative effect of a novel heterozygous G87R mutation identified in women producing zinc-deficient milk. To provide direct visual evidence for the in situ dimerization and function of multiple normal and mutant ZnTs, we applied here the bimolecular fluorescence complementation (BiFC) technique, which enables direct visualization of specific protein-protein interactions. BiFC is based upon reconstitution of an intact fluorescent protein including YFP when its two complementary, non-fluorescent N- and C-terminal fragments (termed YN and YC) are brought together by a pair of specifically interacting proteins. Homodimerization of ZnT1, -2, -3, -4, and -7 was revealed by high subcellular fluorescence observed upon co-transfection of non-fluorescent ZnT-YC and ZnT-YN; this homodimer fluorescence localized in the characteristic compartments of each ZnT. The validity of the BiFC assay in ZnT dimerization was further corroborated when high fluorescence was obtained upon co-transfection of ZnT5-YC and ZnT6-YN, which are known to form heterodimers. We further show that BiFC recapitulated the pathogenic role that ZnT mutations play in transient neonatal zinc deficiency. Zinquin, a fluorescent zinc probe applied along with BiFC, revealed the in situ functionality of ZnT dimers. Hence, the current BiFC-Zinquin technique provides the first in situ evidence for the dimerization and function of wild type and mutant ZnTs in live cells.
Keywords: Metal Homeostasis; Mutant; Protein-Protein Interactions; Transport Metals; Zinc.
Figures





Similar articles
-
Heterodimerization, altered subcellular localization, and function of multiple zinc transporters in viable cells using bimolecular fluorescence complementation.J Biol Chem. 2015 Apr 3;290(14):9050-63. doi: 10.1074/jbc.M114.617332. Epub 2015 Feb 5. J Biol Chem. 2015. PMID: 25657003 Free PMC article.
-
A dominant negative heterozygous G87R mutation in the zinc transporter, ZnT-2 (SLC30A2), results in transient neonatal zinc deficiency.J Biol Chem. 2012 Aug 24;287(35):29348-61. doi: 10.1074/jbc.M112.368159. Epub 2012 Jun 25. J Biol Chem. 2012. PMID: 22733820 Free PMC article. Clinical Trial.
-
Molecular Basis of Transient Neonatal Zinc Deficiency: NOVEL ZnT2 MUTATIONS DISRUPTING ZINC BINDING AND PERMEATION.J Biol Chem. 2016 Jun 24;291(26):13546-59. doi: 10.1074/jbc.M116.732693. Epub 2016 May 2. J Biol Chem. 2016. PMID: 27137936 Free PMC article.
-
Visualization of molecular interactions using bimolecular fluorescence complementation analysis: characteristics of protein fragment complementation.Chem Soc Rev. 2009 Oct;38(10):2876-86. doi: 10.1039/b909638h. Epub 2009 Sep 4. Chem Soc Rev. 2009. PMID: 19771334 Free PMC article. Review.
-
Molecular architecture and function of ZnT transporters.Curr Top Membr. 2012;69:199-220. doi: 10.1016/B978-0-12-394390-3.00008-2. Curr Top Membr. 2012. PMID: 23046652 Review.
Cited by
-
Biological underpinnings of breastfeeding challenges: the role of genetics, diet, and environment on lactation physiology.Am J Physiol Endocrinol Metab. 2016 Aug 1;311(2):E405-22. doi: 10.1152/ajpendo.00495.2015. Epub 2016 Jun 28. Am J Physiol Endocrinol Metab. 2016. PMID: 27354238 Free PMC article. Review.
-
Milk-derived miRNA profiles elucidate molecular pathways that underlie breast dysfunction in women with common genetic variants in SLC30A2.Sci Rep. 2019 Sep 3;9(1):12686. doi: 10.1038/s41598-019-48987-4. Sci Rep. 2019. PMID: 31481661 Free PMC article.
-
Evaluation of the roles of the cytosolic N-terminus and His-rich loop of ZNT proteins using ZNT2 and ZNT3 chimeric mutants.Sci Rep. 2018 Sep 20;8(1):14084. doi: 10.1038/s41598-018-32372-8. Sci Rep. 2018. PMID: 30237557 Free PMC article.
-
The mucolipin-1 (TRPML1) ion channel, transmembrane-163 (TMEM163) protein, and lysosomal zinc handling.Front Biosci (Landmark Ed). 2017 Mar 1;22(8):1330-1343. doi: 10.2741/4546. Front Biosci (Landmark Ed). 2017. PMID: 28199205 Free PMC article. Review.
-
Development of Functional Fluorescent Molecular Probes for the Detection of Biological Substances.Biosensors (Basel). 2015 Jun 18;5(2):337-63. doi: 10.3390/bios5020337. Biosensors (Basel). 2015. PMID: 26095660 Free PMC article. Review.
References
-
- Eide D. J. (2006) Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta 1763, 711–722 - PubMed
-
- Palmiter R. D., Huang L. (2004) Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch. 447, 744–751 - PubMed
-
- Huang L., Tepaamorndech S. (2013) The SLC30 family of zinc transporters. A review of current understanding of their biological and pathophysiological roles. Mol. Aspects Med. 34, 548–560 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

