Inherent regulation of EAL domain-catalyzed hydrolysis of second messenger cyclic di-GMP
- PMID: 24451384
- PMCID: PMC3945359
- DOI: 10.1074/jbc.M113.516195
Inherent regulation of EAL domain-catalyzed hydrolysis of second messenger cyclic di-GMP
Abstract
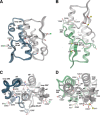
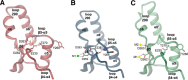
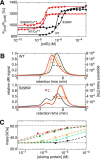
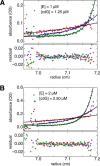
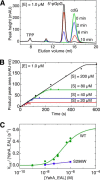
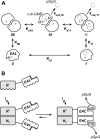
The universal second messenger cyclic di-GMP (cdG) is involved in the regulation of a diverse range of cellular processes in bacteria. The intracellular concentration of the dinucleotide is determined by the opposing actions of diguanylate cyclases and cdG-specific phosphodiesterases (PDEs). Whereas most PDEs have accessory domains that are involved in the regulation of their activity, the regulatory mechanism of this class of enzymes has remained unclear. Here, we use biophysical and functional analyses to show that the isolated EAL domain of a PDE from Escherichia coli (YahA) is in a fast thermodynamic monomer-dimer equilibrium, and that the domain is active only in its dimeric state. Furthermore, our data indicate thermodynamic coupling between substrate binding and EAL dimerization with the dimerization affinity being increased about 100-fold upon substrate binding. Crystal structures of the YahA-EAL domain determined under various conditions (apo, Mg(2+), cdG·Ca(2+) complex) confirm structural coupling between the dimer interface and the catalytic center. The built-in regulatory properties of the EAL domain probably facilitate its modular, functional combination with the diverse repertoire of accessory domains.
Keywords: Allosteric Regulation; Bacterial Signal Transduction; Chromatography; Crystal Structure; Phosphodiesterases; Second Messenger; c-di-GMP Signaling.
Figures



Similar articles
-
The structure of an unconventional HD-GYP protein from Bdellovibrio reveals the roles of conserved residues in this class of cyclic-di-GMP phosphodiesterases.mBio. 2011 Oct 11;2(5):e00163-11. doi: 10.1128/mBio.00163-11. Print 2011. mBio. 2011. PMID: 21990613 Free PMC article.
-
The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: enzymatically active and inactive EAL domains.J Bacteriol. 2005 Jul;187(14):4774-81. doi: 10.1128/JB.187.14.4774-4781.2005. J Bacteriol. 2005. PMID: 15995192 Free PMC article.
-
Formation and dimerization of the phosphodiesterase active site of the Pseudomonas aeruginosa MorA, a bi-functional c-di-GMP regulator.FEBS Lett. 2014 Dec 20;588(24):4631-6. doi: 10.1016/j.febslet.2014.11.002. Epub 2014 Nov 11. FEBS Lett. 2014. PMID: 25447517
-
Trigger phosphodiesterases as a novel class of c-di-GMP effector proteins.Philos Trans R Soc Lond B Biol Sci. 2016 Nov 5;371(1707):20150498. doi: 10.1098/rstb.2015.0498. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27672149 Free PMC article. Review.
-
C-di-GMP Synthesis: Structural Aspects of Evolution, Catalysis and Regulation.J Mol Biol. 2016 Sep 25;428(19):3683-701. doi: 10.1016/j.jmb.2016.07.023. Epub 2016 Aug 4. J Mol Biol. 2016. PMID: 27498163 Review.
Cited by
-
Screening for Diguanylate Cyclase (DGC) Inhibitors Mitigating Bacterial Biofilm Formation.Front Chem. 2020 Apr 21;8:264. doi: 10.3389/fchem.2020.00264. eCollection 2020. Front Chem. 2020. PMID: 32373581 Free PMC article. Review.
-
Genome-Based Comparison of Cyclic Di-GMP Signaling in Pathogenic and Commensal Escherichia coli Strains.J Bacteriol. 2015 Aug 24;198(1):111-26. doi: 10.1128/JB.00520-15. Print 2016 Jan 1. J Bacteriol. 2015. PMID: 26303830 Free PMC article.
-
The Characterization of Escherichia coli CpdB as a Recombinant Protein Reveals that, besides Having the Expected 3´-Nucleotidase and 2´,3´-Cyclic Mononucleotide Phosphodiesterase Activities, It Is Also Active as Cyclic Dinucleotide Phosphodiesterase.PLoS One. 2016 Jun 13;11(6):e0157308. doi: 10.1371/journal.pone.0157308. eCollection 2016. PLoS One. 2016. PMID: 27294396 Free PMC article.
-
Characterisation of sequence-structure-function space in sensor-effector integrators of phytochrome-regulated diguanylate cyclases.Photochem Photobiol Sci. 2022 Oct;21(10):1761-1779. doi: 10.1007/s43630-022-00255-7. Epub 2022 Jul 5. Photochem Photobiol Sci. 2022. PMID: 35788917 Free PMC article.
-
Regulation of Exopolysaccharide Production by ProE, a Cyclic-Di-GMP Phosphodiesterase in Pseudomonas aeruginosa PAO1.Front Microbiol. 2020 Jun 5;11:1226. doi: 10.3389/fmicb.2020.01226. eCollection 2020. Front Microbiol. 2020. PMID: 32582123 Free PMC article.
References
-
- Hengge R. (2009) Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 7, 263–273 - PubMed
-
- Wassmann P., Chan C., Paul R., Beck A., Heerklotz H., Jenal U., Schirmer T. (2007) Structure of BeF3-modified response regulator PleD. Implications for diguanylate cyclase activation, catalysis, and feedback inhibition. Structure 15, 915–927 - PubMed
-
- Paul R., Abel S., Wassmann P., Beck A., Heerklotz H., Jenal U. (2007) Activation of the diguanylate cyclase PleD by phosphorylation-mediated dimerization. J. Biol. Chem. 282, 29170–29177 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

