Active immunity induced by passive IgG post-exposure protection against ricin
- PMID: 24451844
- PMCID: PMC3920268
- DOI: 10.3390/toxins6010380
Active immunity induced by passive IgG post-exposure protection against ricin
Abstract
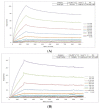
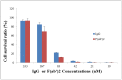
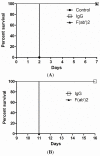
Therapeutic antibodies can confer an instant protection against biothreat agents when administered. In this study, intact IgG and F(ab')2 from goat anti-ricin hyperimmune sera were compared for the protection against lethal ricin mediated intoxication. Similar ricin-binding affinities and neutralizing activities in vitro were observed between IgG and F(ab')2 when compared at the same molar concentration. In a murine ricin intoxication model, both IgG and F(ab')2 could rescue 100% of the mice by one dose (3 nmol) administration of antibodies 1 hour after 5 × LD50 ricin challenge. Nine days later, when the rescued mice received a second ricin challenge (5 × LD50), only the IgG-treated mice survived; the F(ab')2-treated mice did not. The experimental design excluded the possibility of residual goat IgG responsible for the protection against the second ricin challenge. Results confirmed that the active immunity against ricin in mice was induced quickly following the passive delivery of a single dose of goat IgG post-exposure. Furthermore, it was demonstrated that the induced active immunity against ricin in mice lasted at least 5 months. Therefore, passive IgG therapy not only provides immediate protection to the victim after ricin exposure, but also elicits an active immunity against ricin that subsequently results in long term protection.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

