Testicular development in male rats is sensitive to a soy-based diet in the neonatal period
- PMID: 24451983
- PMCID: PMC4076408
- DOI: 10.1095/biolreprod.113.113787
Testicular development in male rats is sensitive to a soy-based diet in the neonatal period
Abstract
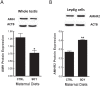
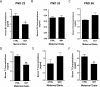
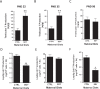
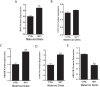
Approximately 30% of infants in the United States are exposed to high doses of isoflavones resulting from soy infant formula consumption. Soybeans contain the isoflavones genistin and daidzin, which are hydrolyzed in the gastrointestinal tract to their genistein and daidzein aglycones. Both aglycones possess hormonal activity and may interfere with male reproductive development. Testosterone, which supports male fertility, is mainly produced by testicular Leydig cells. Our previous studies indicated that perinatal exposure of male rats to isoflavones induced proliferative activity in Leydig cells and increased testosterone concentrations into adulthood. However, the relevance of the neonatal period as part of the perinatal window of isoflavone exposure remains to be established. The present study examined the effects of exposure to isoflavones on male offspring of dams maintained on a casein-based control or whole soybean diet in the neonatal period, that is, Days 2 to 21 postpartum. The results showed that the soybean diet stimulated proliferative activity in developing Leydig cells while suppressing their steroidogenic capacity in adulthood. In addition, isoflavone exposure decreased production of anti-Müllerian hormone by Sertoli cells. Similar to our previous in vitro studies of genistein action in Leydig cells, daidzein induced proliferation and interfered with signaling pathways to suppress steroidogenic activity. Overall, the data showed that the neonatal period is a sensitive window of exposure to isoflavones and support the view that both genistein and daidzein are responsible for biological effects associated with soy-based diets.
Keywords: Leydig cells; androgen; androgens/androgen receptor; daidzein; endocrine disruptors; genistein; phytoestrogen; sex steroids; testis; toxicology.
Figures



Similar articles
-
Regulation of the neuroendocrine axis in male rats by soy-based diets is independent of age and due specifically to isoflavone action†.Biol Reprod. 2020 Oct 5;103(4):892-906. doi: 10.1093/biolre/ioaa101. Biol Reprod. 2020. PMID: 32520353 Free PMC article.
-
Exposure to phytoestrogens in the perinatal period affects androgen secretion by testicular Leydig cells in the adult rat.Endocrinology. 2007 Sep;148(9):4475-88. doi: 10.1210/en.2007-0327. Epub 2007 Jun 14. Endocrinology. 2007. PMID: 17569756
-
Developmental exposures of male rats to soy isoflavones impact Leydig cell differentiation.Biol Reprod. 2010 Sep;83(3):488-501. doi: 10.1095/biolreprod.109.082685. Epub 2010 Jun 10. Biol Reprod. 2010. PMID: 20554919 Free PMC article.
-
Changes in MAPK pathway in neonatal and adult testis following fetal estrogen exposure and effects on rat testicular cells.Microsc Res Tech. 2009 Nov;72(11):773-86. doi: 10.1002/jemt.20756. Microsc Res Tech. 2009. PMID: 19565636 Review.
-
Soy Isoflavones and their Effects on Xenobiotic Metabolism.Curr Drug Metab. 2019;20(1):46-53. doi: 10.2174/1389200219666180427170213. Curr Drug Metab. 2019. PMID: 29708073 Review.
Cited by
-
EB 2017 Article: Soy protein isolate feeding does not result in reproductive toxicity in the pre-pubertal rat testis.Exp Biol Med (Maywood). 2018 May;243(8):695-707. doi: 10.1177/1535370218771333. Exp Biol Med (Maywood). 2018. PMID: 29763383 Free PMC article.
-
Effects of soy isoflavones on testosterone synthetase in diet-induced obesity male rats.Int J Clin Exp Pathol. 2017 Sep 1;10(9):9202-9212. eCollection 2017. Int J Clin Exp Pathol. 2017. PMID: 31966792 Free PMC article.
-
Estrogenic Pastures: A Source of Endocrine Disruption in Sheep Reproduction.Front Endocrinol (Lausanne). 2022 Apr 28;13:880861. doi: 10.3389/fendo.2022.880861. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35574027 Free PMC article. Review.
-
Assessment of safety and efficacy of perinatal or peripubertal exposure to daidzein on bone development in rats.Toxicol Rep. 2015 Jan 2;2:429-436. doi: 10.1016/j.toxrep.2014.12.012. eCollection 2015. Toxicol Rep. 2015. PMID: 28962378 Free PMC article.
-
Regulation of the neuroendocrine axis in male rats by soy-based diets is independent of age and due specifically to isoflavone action†.Biol Reprod. 2020 Oct 5;103(4):892-906. doi: 10.1093/biolre/ioaa101. Biol Reprod. 2020. PMID: 32520353 Free PMC article.
References
-
- Akingbemi BT, Braden TD, Kemppainen BW, Hancock KD, Sherrill JD, Cook SJ, He X, Supko JG. Exposure to phytoestrogens in the perinatal period affects androgen secretion by testicular Leydig cells in the adult rat. Endocrinology 2007; 148: 4475–4488. - PubMed
-
- Sharpe RM, Martin B, Morris K, Greig I, McKinnell C, McNeilly AS, Walker M. Infant feeding with soy formula milk: effects on the testis and on blood testosterone levels in marmoset monkeys during the period of neonatal testicular activity. Hum Reprod 2002; 17: 1692–1703. - PubMed
-
- Wisniewski AB, Klein SL, Lakshmanan Y, Gearhart JP. Exposure to genistein during gestation and lactation demasculinizes the reproductive system in rats. J Urol 2003; 169: 1582–1586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

