Actomyosin-dependent formation of the mechanosensitive talin-vinculin complex reinforces actin anchoring
- PMID: 24452080
- PMCID: PMC3916842
- DOI: 10.1038/ncomms4095
Actomyosin-dependent formation of the mechanosensitive talin-vinculin complex reinforces actin anchoring
Abstract
The force generated by the actomyosin cytoskeleton controls focal adhesion dynamics during cell migration. This process is thought to involve the mechanical unfolding of talin to expose cryptic vinculin-binding sites. However, the ability of the actomyosin cytoskeleton to directly control the formation of a talin-vinculin complex and the resulting activity of the complex are not known. Here we develop a microscopy assay with pure proteins in which the self-assembly of actomyosin cables controls the association of vinculin to a talin-micropatterned surface in a reversible manner. Quantifications indicate that talin refolding is limited by vinculin dissociation and modulated by the actomyosin network stability. Finally, we show that the activation of vinculin by stretched talin induces a positive feedback that reinforces the actin-talin-vinculin association. This in vitro reconstitution reveals the mechanism by which a key molecular switch senses and controls the connection between adhesion complexes and the actomyosin cytoskeleton.
Figures

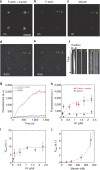
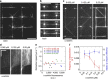
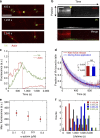
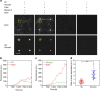

References
-
- Wehrle-Haller B. Structure and function of focal adhesions. Curr. Opin. Cell Biol. 24, 116–124 (2012). - PubMed
-
- Zaidel-Bar R., Ballestrem C., Kam Z. & Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci. 116, 4605–4613 (2003). - PubMed
-
- Geiger B., Spatz J. P. & Bershadsky A. D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 10, 21–33 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

