Cryoimmunotherapy with local co-administration of ex vivo generated dendritic cells and CpG-ODN immune adjuvant, elicits a specific antitumor immunity
- PMID: 24452202
- PMCID: PMC11029716
- DOI: 10.1007/s00262-014-1520-4
Cryoimmunotherapy with local co-administration of ex vivo generated dendritic cells and CpG-ODN immune adjuvant, elicits a specific antitumor immunity
Abstract
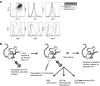
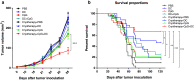
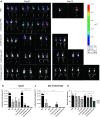
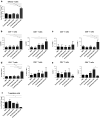
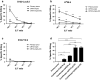
Cryoablation is a low-invasive surgical procedure for management of malignant tumors. Tissue destruction is obtained by repeated deep freezing and thawing and results in coagulative necrosis and in apoptosis. This procedure induces the release of tumor-associated antigens and proinflammatory factors into the microenvironment. Local administration of immature dendritic cells (DCs) potentiates the immune response induced by cryoablation. To further augment the induction of long-lasting antitumor immunity, we investigated the clinical value of combining cryoimmunotherapy consisting of cryoablation and inoculation of immature DCs with administration of the immune adjuvant, CpG oligodeoxynucleotides. Injection of the murine Lewis lung carcinoma, D122-luc-5.5 that expresses the luciferase gene, results in spontaneous metastases, which can be easily monitored in vivo. The local tumor was treated by the combined treatment. The clinical outcome was assessed by monitoring tumor growth, metastasis in distant organs, overall survival, and protection from tumor recurrence. The nature of the induced T cell responses was analyzed. Combined cryoimmunotherapy results in reduced tumor growth, low metastasis and significantly prolonged survival. Moreover, this treatment induces antitumor memory that protected mice from rechallenge. The underlying suggested mechanisms are the generation of tumor-specific type 1 T cell responses, subsequent induction of cytotoxic T lymphocytes, and generation of systemic memory. Our data highlight the combined cryoimmunotherapy as a novel antitumor vaccine with promising preclinical results. Adjustment of this technique into practice will provide the therapeutic benefits of both, ablation of the primary tumor and induction of robust antitumor and antimetastatic immunity.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

