Gene therapy prolongs survival and restores function in murine and canine models of myotubular myopathy
- PMID: 24452262
- PMCID: PMC4105197
- DOI: 10.1126/scitranslmed.3007523
Gene therapy prolongs survival and restores function in murine and canine models of myotubular myopathy
Abstract
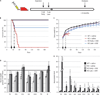
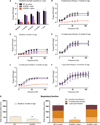
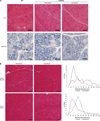
Loss-of-function mutations in the myotubularin gene (MTM1) cause X-linked myotubular myopathy (XLMTM), a fatal, congenital pediatric disease that affects the entire skeletal musculature. Systemic administration of a single dose of a recombinant serotype 8 adeno-associated virus (AAV8) vector expressing murine myotubularin to Mtm1-deficient knockout mice at the onset or at late stages of the disease resulted in robust improvement in motor activity and contractile force, corrected muscle pathology, and prolonged survival throughout a 6-month study. Similarly, single-dose intravascular delivery of a canine AAV8-MTM1 vector in XLMTM dogs markedly improved severe muscle weakness and respiratory impairment, and prolonged life span to more than 1 year in the absence of toxicity or a humoral or cell-mediated immune response. These results demonstrate the therapeutic efficacy of AAV-mediated gene therapy for myotubular myopathy in small- and large-animal models, and provide proof of concept for future clinical trials in XLMTM patients.
Figures




References
-
- Bainbridge JW, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008 May 22;358:2231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

