Chronic spinal cord electrical stimulation protects against 6-hydroxydopamine lesions
- PMID: 24452435
- PMCID: PMC3899601
- DOI: 10.1038/srep03839
Chronic spinal cord electrical stimulation protects against 6-hydroxydopamine lesions
Abstract
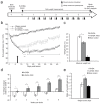
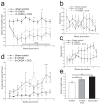
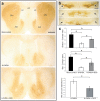
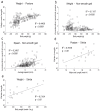
Although L-dopa continues to be the gold standard for treating motor symptoms of Parkinson's disease (PD), it presents long-term complications. Deep brain stimulation is effective, but only a small percentage of idiopathic PD patients are eligible. Based on results in animal models and a handful of patients, dorsal column stimulation (DCS) has been proposed as a potential therapy for PD. To date, the long-term effects of DCS in animal models have not been quantified. Here, we report that DCS applied twice a week in rats treated with bilateral 6-OHDA striatal infusions led to a significant improvement in symptoms. DCS-treated rats exhibited a higher density of dopaminergic innervation in the striatum and higher neuronal cell count in the substantia nigra pars compacta compared to a control group. These results suggest that DCS has a chronic therapeutical and neuroprotective effect, increasing its potential as a new clinical option for treating PD patients.
Figures

References
-
- Carlsson A. Biochemical and pharmacological aspects of Parkinsonism. Acta Neurol. Scand. Suppl. 51, 11–42 (1972). - PubMed
-
- Nagatsua T. & Sawadab M. L-dopa therapy for Parkinson's disease: past, present, and future. Parkinsonism Relat Disord 15 Suppl 1, S3–8, 10.1016/S1353-8020(09)70004-5 (2009). - PubMed
-
- Benabid A. L. Deep brain stimulation for Parkinson's disease. Curr. Opin. Neurobiol. 13, 696–706 (2003). - PubMed
-
- Morgante L. et al. How many parkinsonian patients are suitable candidates for deep brain stimulation of subthalamic nucleus? Results of a questionnaire. Parkinsonism Relat Disord 13, 528–531, 10.1016/j.parkreldis.2006.12.013 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

