Regulation of GluA1 α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor function by protein kinase C at serine-818 and threonine-840
- PMID: 24452473
- PMCID: PMC4014661
- DOI: 10.1124/mol.113.091488
Regulation of GluA1 α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor function by protein kinase C at serine-818 and threonine-840
Abstract
Three residues within the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor subunit GluA1 C terminus (Ser818, Ser831, Thr840) can be phosphorylated by Ca(2+)/phospholipid-dependent protein kinase (PKC). Here, we show that PKC phosphorylation of GluA1 Ser818 or Thr840 enhances the weighted mean channel conductance without altering the response time course or agonist potency. These data support the idea that these residues constitute a hyper-regulatory domain for the AMPA receptor. Introduction of phosphomimetic mutations increases conductance only at these three sites within the proximal C terminus, consistent with a structural model with a flexible linker connecting the distal C-terminal domain to the more proximal domain containing a helix bracketed by Ser831 and Thr840. NMR spectra support this model and raise the possibility that phosphorylation can alter the configuration of this domain. Our findings provide insight into the structure and function of the C-terminal domain of GluA1, which controls AMPA receptor function and trafficking during synaptic plasticity in the central nervous system.
Figures




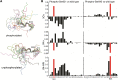
 where ΔN is the difference in the nitrogen shift and ΔHN is the shift in the nitrogen proton.
where ΔN is the difference in the nitrogen shift and ΔHN is the shift in the nitrogen proton.References
-
- Barria A, Derkach V, Soderling T. (1997a) Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem 272:32727–32730 - PubMed
-
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. (1997b) Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science 276:2042–2045 - PubMed
-
- Benke TA, Lüthi A, Isaac JT, Collingridge GL. (1998) Modulation of AMPA receptor unitary conductance by synaptic activity. Nature 393:793–797 - PubMed
-
- Bliss TV, Collingridge GL. (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31–39 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

