Patient-specific academic detailing for smoking cessation: feasibility study
- PMID: 24452574
- PMCID: PMC3994822
Patient-specific academic detailing for smoking cessation: feasibility study
Abstract
Objective: To describe and to determine the feasibility of a patient-specific academic detailing (PAD) smoking cessation (SC) program in a primary care setting.
Design: Descriptive cohort feasibility study.
Setting: Hamilton, Ont.
Participants: Pharmacists, physicians, nurse practitioners, and their patients.
Interventions: Integrated pharmacists received basic academic detailing training and education on SC and then delivered PAD to prescribers using structured verbal education and written materials. Data were collected using structured forms.
Main outcome measures: Five main feasibility criteria were generated based on Canadian academic detailing programs: PAD coordinator time to train pharmacists less than 40 hours; median time of SC education per pharmacist less than 20 hours; median time per PAD session less than 60 minutes for initial visit; percentage of prescribers receiving PAD within 3 months greater than 50%; and number of new SC referrals to pharmacists at 6 months more than 10 patients per 1.0 full-time equivalent (FTE) pharmacist (total of approximately 30 patients).
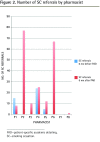
Results: Eight pharmacists (5.8 FTE) received basic academic detailing training and education on SC PAD. Forty-eight physicians and 9 nurse practitioners consented to participate in the study. The mean PAD coordinator training time was 29.1 hours. The median time for SC education was 3.1 hours. The median times for PAD sessions were 15 and 25 minutes for an initial visit and follow-up visit, respectively. The numbers of prescribers who had received PAD at 3 and 6 months were 50 of 64 (78.1%) and 57 of 64 (89.1%), respectively. The numbers of new SC referrals at 3 and 6 months were 11 patients per FTE pharmacist (total of 66 patients) and 34 patients per FTE pharmacist (total of 200 patients), respectively.
Conclusion: This study met the predetermined feasibility criteria with respect to the management, resources, process, and scientific components. Further study is warranted to determine whether PAD is more effective than conventional academic detailing.
Objectif: Décrire un programme de formation continue en pharmacothérapie spécifique au patient (FPSP) pour la cessation du tabagisme (CT) et en déterminer la faisabilité dans un milieu de soins primaires.
Type d’étude: Étude de cohorte descriptive sur la faisabilité.
Contexte: Hamilton, en Ontario.
Participants: Des pharmaciens, des médecins, des infirmières praticiennes et leurs patients.
Interventions: Un groupe intégré de pharmaciens a suivi une formation de base en pharmacothérapie et un programme d’éducation en CT, puis a présenté cette même formation aux prescripteurs sous forme verbale structurée et à l’aide de matériel écrit. Les données ont été recueillies au moyen de formulaires structurés.
Principaux paramètres à l’étude: On a élaboré cinq principaux critères de faisabilité en se fondant sur les programmes de formation continue en pharmacothérapie canadiens: moins de 40 heures consacrées par le coordonnateur du programme de FPSP pour former les pharmaciens; moins de 20 heures en moyenne par pharmacien pour la formation en CT; moins de 60 minutes en moyenne par séance de FPSP pour la visite initiale; plus de 50 % des prescripteurs suivant un programme de FPSP dans un délai de 3 mois; et un nombre supérieur à 10 nouveaux patients par 1,0 pharmacien équivalent temps plein (ETP) envoyés en consultation pour CT (total d’environ 30 patients).
Résultats: Huit pharmaciens (5,8 ETP) ont reçu une formation en pharmacothérapie continue de base et ont suivi un programme d’éducation en CT. Quarante-huit médecins et 9 infirmières praticiennes ont consenti à participer à l’étude. Le coordonnateur du programme de FPSP a consacré en moyenne 29,1 heures pour la formation. La durée moyenne pour la formation en CT était de 3,1 heures. La visite initiale et celle de suivi pour les séances du FPSP duraient respectivement 15 et 25 minutes. Les nombres de prescripteurs ayant suivi la FPSP à 3 et à 6 mois se situaient à 50 sur 64 (78,1 %) et à 57 sur 64 (89,1 %) respectivement. Les nombres de nouvelles demandes de consultation en CT à 3 et à 6 mois s’élevaient respectivement à 11 patients par pharmacien ETP (total de 66 patients) et à 34 patients par pharmacien ETP (total de 200 patients) respectivement.
Conclusion: Cette étude a satisfait aux critères de faisabilité prédéterminés en ce qui concerne la gestion, les ressources, le processus et les composantes scientifiques. Des études plus approfondies s’imposent pour déterminer si la FPSP est plus efficace que la formation continue en pharmacothérapie conventionnelle.
Figures
Similar articles
-
Pharmacist-led academic detailing intervention in primary care: a mixed methods feasibility study.Int J Clin Pharm. 2019 Apr;41(2):574-582. doi: 10.1007/s11096-019-00787-6. Epub 2019 Jan 22. Int J Clin Pharm. 2019. PMID: 30666611
-
Technology-enabled academic detailing: computer-mediated education between pharmacists and physicians for evidence-based prescribing.Int J Med Inform. 2013 Sep;82(9):762-71. doi: 10.1016/j.ijmedinf.2013.04.011. Epub 2013 Jun 14. Int J Med Inform. 2013. PMID: 23770028
-
Academic Detailing Interventions Improve Tobacco Use Treatment among Physicians Working in Underserved Communities.Ann Am Thorac Soc. 2015 Jun;12(6):854-8. doi: 10.1513/AnnalsATS.201410-466BC. Ann Am Thorac Soc. 2015. PMID: 25867533 Free PMC article.
-
Improving the success of mailed letter intervention programs to influence prescribing behaviors: a review.J Manag Care Pharm. 2012 Oct;18(8):627-49. doi: 10.18553/jmcp.2012.18.8.627. J Manag Care Pharm. 2012. PMID: 23127150 Free PMC article. Review.
-
Potentially Inappropriate Prescribing to Older Patients: Criteria, Prevalence and an Intervention to Reduce It: The Prescription Peer Academic Detailing (Rx-PAD) Study - A Cluster-Randomized, Educational Intervention in Norwegian General Practice.Basic Clin Pharmacol Toxicol. 2018 Oct;123(4):380-391. doi: 10.1111/bcpt.13040. Epub 2018 Jun 21. Basic Clin Pharmacol Toxicol. 2018. PMID: 29753315 Review.
Cited by
-
Enhancing Naloxone Distribution for Opioid Users in the USA: A Cost-Utility Analysis of Academic Detailing to Clinicians.Appl Health Econ Health Policy. 2025 Jul 20. doi: 10.1007/s40258-025-00991-8. Online ahead of print. Appl Health Econ Health Policy. 2025. PMID: 40685487
-
Can Academic Detailing Move the Needle for Patients with Diabetes in a State-Based Prescription Drug Benefit Program?Am Health Drug Benefits. 2019 Apr;12(2):94-102. Am Health Drug Benefits. 2019. PMID: 31057695 Free PMC article.
-
Key Features of Academic Detailing: Development of an Expert Consensus Using the Delphi Method.Am Health Drug Benefits. 2016 Feb;9(1):42-50. Am Health Drug Benefits. 2016. PMID: 27066195 Free PMC article.
-
From Good to Great: The Role of Performance Coaching in Enhancing Tobacco-Dependence Treatment Rates.Ann Fam Med. 2018 Nov;16(6):498-506. doi: 10.1370/afm.2312. Ann Fam Med. 2018. PMID: 30420364 Free PMC article. Clinical Trial.
References
-
- KT knowledge base. Glossary. Ottawa, ON: KT Clearinghouse; 2006. Knowledge Translation Program. Available from: http://ktclearinghouse.ca/knowledgebase/glossary. Accessed 2013 Dec 13.
-
- Grimshaw JM, Shirran L, Thomas R, Mowatt G, Fraser C, Bero L, et al. Changing provider behavior: an overview of systematic reviews of interventions. Med Care. 2001;39(8 Suppl 2):II2–45. - PubMed
-
- Coenen S, Van Royen P, Michiels B, Denekens J. Optimizing antibiotic prescribing for acute cough in general practice: a cluster-randomized controlled trial. J Antimicrob Chemother. 2004;54(3):661–72. Epub 2004 Jul 28. - PubMed
-
- Seager JM, Howell-Jones RS, Dunstan FD, Lewis MA, Richmond S, Thomas DW. A randomised controlled trial of clinical outreach education to rationalise antibiotic prescribing for acute dental pain in the primary care setting. Br Dent J. 2006;201(4):217–22. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous