Genetic validation of the protein arginine methyltransferase PRMT5 as a candidate therapeutic target in glioblastoma
- PMID: 24453002
- PMCID: PMC3959215
- DOI: 10.1158/0008-5472.CAN-13-0884
Genetic validation of the protein arginine methyltransferase PRMT5 as a candidate therapeutic target in glioblastoma
Abstract
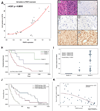
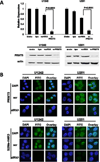
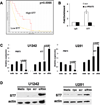
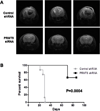
Glioblastoma is the most common and aggressive histologic subtype of brain cancer with poor outcomes and limited treatment options. Here, we report the selective overexpression of the protein arginine methyltransferase PRMT5 as a novel candidate theranostic target in this disease. PRMT5 silences the transcription of regulatory genes by catalyzing symmetric dimethylation of arginine residues on histone tails. PRMT5 overexpression in patient-derived primary tumors and cell lines correlated with cell line growth rate and inversely with overall patient survival. Genetic attenuation of PRMT5 led to cell-cycle arrest, apoptosis, and loss of cell migratory activity. Cell death was p53-independent but caspase-dependent and enhanced with temozolomide, a chemotherapeutic agent used as a present standard of care. Global gene profiling and chromatin immunoprecipitation identified the tumor suppressor ST7 as a key gene silenced by PRMT5. Diminished ST7 expression was associated with reduced patient survival. PRMT5 attenuation limited PRMT5 recruitment to the ST7 promoter, led to restored expression of ST7 and cell growth inhibition. Finally, PRMT5 attenuation enhanced glioblastoma cell survival in a mouse xenograft model of aggressive glioblastoma. Together, our findings defined PRMT5 as a candidate prognostic factor and therapeutic target in glioblastoma, offering a preclinical justification for targeting PRMT5-driven oncogenic pathways in this deadly disease.
©2014 AACR.
Figures



Similar articles
-
Targeting protein arginine methyltransferase 5 inhibits colorectal cancer growth by decreasing arginine methylation of eIF4E and FGFR3.Oncotarget. 2015 Sep 8;6(26):22799-811. doi: 10.18632/oncotarget.4332. Oncotarget. 2015. PMID: 26078354 Free PMC article.
-
Protein arginine methyltransferase 5-mediated epigenetic silencing of IRX1 contributes to tumorigenicity and metastasis of gastric cancer.Biochim Biophys Acta Mol Basis Dis. 2018 Sep;1864(9 Pt B):2835-2844. doi: 10.1016/j.bbadis.2018.05.015. Epub 2018 May 23. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 29802960
-
Protein arginine methyltransferase 5 functions as an epigenetic activator of the androgen receptor to promote prostate cancer cell growth.Oncogene. 2017 Mar 2;36(9):1223-1231. doi: 10.1038/onc.2016.287. Epub 2016 Aug 22. Oncogene. 2017. PMID: 27546619 Free PMC article.
-
Aberrant miRNA expression and protein arginine methyltransferase 5 (PRMT5) in cancer: A review.Biochim Biophys Acta Mol Cell Res. 2025 Mar;1872(3):119923. doi: 10.1016/j.bbamcr.2025.119923. Epub 2025 Feb 22. Biochim Biophys Acta Mol Cell Res. 2025. PMID: 39993609 Review.
-
Protein arginine N-methyltransferase 5 in colorectal carcinoma: Insights into mechanisms of pathogenesis and therapeutic strategies.Biomed Pharmacother. 2022 Jan;145:112368. doi: 10.1016/j.biopha.2021.112368. Epub 2021 Nov 15. Biomed Pharmacother. 2022. PMID: 34794114 Review.
Cited by
-
PRMT6 facilitates EZH2 protein stability by inhibiting TRAF6-mediated ubiquitination degradation to promote glioblastoma cell invasion and migration.Cell Death Dis. 2024 Jul 23;15(7):524. doi: 10.1038/s41419-024-06920-2. Cell Death Dis. 2024. PMID: 39043634 Free PMC article.
-
Multi-Scale Spatial Analysis of the Tumor Microenvironment Reveals Features of Cabozantinib and Nivolumab Efficacy in Hepatocellular Carcinoma.Front Immunol. 2022 May 13;13:892250. doi: 10.3389/fimmu.2022.892250. eCollection 2022. Front Immunol. 2022. PMID: 35634309 Free PMC article.
-
Clinicopathological and Prognostic Significance of PRMT5 in Cancers: A System Review and Meta-Analysis.Cancer Control. 2021 Jan-Dec;28:10732748211050583. doi: 10.1177/10732748211050583. Cancer Control. 2021. PMID: 34758643 Free PMC article.
-
Targeting protein arginine methyltransferase 5 inhibits human hepatocellular carcinoma growth via the downregulation of beta-catenin.J Transl Med. 2015 Nov 5;13:349. doi: 10.1186/s12967-015-0721-8. J Transl Med. 2015. PMID: 26541651 Free PMC article.
-
PRMT5-PTEN molecular pathway regulates senescence and self-renewal of primary glioblastoma neurosphere cells.Oncogene. 2017 Jan 12;36(2):263-274. doi: 10.1038/onc.2016.199. Epub 2016 Jun 13. Oncogene. 2017. PMID: 27292259 Free PMC article.
References
-
- Walker MD, Green SB, Byar DP, Alexander E, Jr, Batzdorf U, Brooks WH, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303:1323–1329. - PubMed
-
- Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol. 2007;25:4127–4136. - PubMed
-
- Karberg S. Switching on epigenetic therapy. Cell. 2009;139:1029–1031. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

