Yellow fever vaccine for patients with HIV infection
- PMID: 24453061
- PMCID: PMC11245961
- DOI: 10.1002/14651858.CD010929.pub2
Yellow fever vaccine for patients with HIV infection
Abstract
Background: Yellow fever (YF) is an acute viral haemorrhagic disease prevalent in tropical Africa and Latin America. The World Health Organization (WHO) estimates that there are 200,000 cases of YF and 30,000 deaths worldwide annually. Treatment for YF is supportive, but a live attenuated virus vaccine is effective for preventing infection. WHO recommends immunisation for all individuals > 9 months living in countries or areas at risk. However, the United States Advisory Committee on Immunization Practices (ACIP) advises that YF vaccine is contraindicated in individuals with HIV. Given the large populations of HIV-infected individuals living in tropical areas where YF is endemic, YF vaccine may be an important intervention for preventing YF in immunocompromised populations.
Objectives: To assess the risk and benefits of YF immunisation for people infected with HIV.
Search methods: We used standard Cochrane methods to search electronic databases and conference proceedings with relevant search terms without limits to language.
Selection criteria: Randomised controlled trials and cohort studies of individuals with HIV infection who received YF vaccine (17DD or 17D-204).
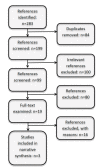
Data collection and analysis: Two authors screened abstracts of references identified by electronic or bibliographic searches according to inclusion and exclusion criteria as detailed in the protocol. We identified 199 references and examined 19 in detail for study eligibility. Data were abstracted independently using a standardised abstraction form.
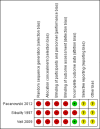
Main results: Three cohort studies were included in the review. They examined 484 patients with HIV infection who received YF immunisation. Patients with HIV infection developed significantly lower concentrations of neutralising antibodies in the first year post immunisation compared to uninfected patients, though decay patterns were similar for recipients regardless of HIV infection. No study patient with HIV infection suffered serious adverse events as a result of YF vaccination.
Authors' conclusions: YF vaccination can produce protective levels of neutralising antibodies in HIV patients. Immunogenicity of YF vaccine is slightly less in HIV-infected patients compared to HIV-uninfected patients. No serious adverse events related to YF vaccine were observed in HIV-infected study participants. At time of immunisation, higher CD4 cell counts and lower HIV RNA levels in patients with HIV infection seem to be key determinants for development of protective titres of neutralising antibodies. The quality of the evidence for all outcomes was low to very low. YF vaccine may potentially be used safely in HIV-infected patients, although our conclusions are limited by small numbers of patients who have been reported. To assure maximum effectiveness YF vaccine should be given to HIV-infected patients after HIV replication has been suppressed.
Conflict of interest statement
None known.
Figures





Update of
References
References to studies included in this review
Pacanowski 2012 {published data only}
-
- Pacanowski J, Lacombe K, Campa P, Dabrowska M, Poveda JD, Meynard JL, Poirot JL, Fonquernie L, Girard PM. Plasma HIV‐RNA is the key determinant of long‐term antibody persistence after yellow fever immunization in a cohort of 364 HIV‐infected patients. J Acquir Immune Defic Syndr. 2012 Apr 1;59(4):360‐67. - PubMed
Sibailly 1997 {published data only}
-
- Sibailly TS, Wiktor SZ, Tsai TF, Cropp BC, Ekpini ER, Adjorlolo‐Johnson G, Gnaore E, et al. Poor antibody response to yellow fever vaccination in children infected with human immunodeficiency virus type 1. Pediatr Infect Dis J. 1997 Dec;16(12):1177‐79. - PubMed
Veit 2009 {published data only}
-
- Veit O, Niedrig M, Chapuis‐Taillard C, Cavassini M, Mossdorf E, Schmid P, Bae H‐G, Litzba N, Staub T, Hatz C, Furrer H, Swiss HIV Cohort Study. Immunogenicity and safety of yellow fever vaccination for 102 HIV‐infected patients. Clin Infect Dis. 2009 Mar 1;48(5):659‐66. - PubMed
References to studies excluded from this review
Camacho 2004 {published data only}
-
- Camacho LA, Freire Mda S, Leal Mda L, Aguiar SG, Nascimento JP, Iguchi T, et al. Immunogenicity of WHO‐17D and Brazilian 17DD yellow fever vaccines: a randomized trial. Rev Saude Publica. 2004 Oct;38(5):671‐8. - PubMed
Camacho 2007 {published data only}
-
- Collaborative Group for Studies with Yellow Fever Vaccine. Randomized, double‐blind, multicenter study of the immunogenicity and reactogenicity of 17DD and WHO 17D‐213/77 yellow fever vaccines in children: implications for the Brazilian National Immunization Program. Vaccine. 2007 Apr 20;25(16):3118‐23. - PubMed
Goujon 1995 {published data only}
-
- Goujon C, Tohr M, Feuillie V, Coulaud P, Dupont B, Sansonetti P. Good tolerance and efficacy of yellow fever vaccine among carriers of human immunodeficiency virus. J Trav Med 1995;2:145.
Ho 2008 {published data only}
-
- Ho YL, Enohata T, Lopes MH, Sousa Dos Santos S. Vaccination in Brazilian HIV‐infected adults: a cross‐sectional study. AIDS Patient Care STDs. 2008 Jan;22(1):65‐70. - PubMed
Kengsakul 2002 {published data only}
-
- Kengsakul, K, Sathirapongsasuti K, Punyagupta S. Fatal myeloencephalitis following yellow fever vaccination in a case with HIV infection. J Med Assoc Thailand 2002;85:131‐4. - PubMed
Monath 2002 {published data only}
-
- Monath TP, Nichols R, Archambault WT, Moore L, Marchesani R, Tian J, et al. Comparative safety and immunogenicity of two yellow fever 17D vaccines (ARILVAX and YF‐VAX) in a phase III multicenter, double‐blind clinical trial. Am J Trop Med Hyg. 2002 May;66(5):533‐41. - PubMed
Osinusi 1990 {published data only}
-
- Osinusi K, Akinkugbe FM, Akinwolere OA, Fabiyi A. Safety and efficacy of yellow fever vaccine in children less than one‐year‐old. West Afr J Med. 1990 Jul‐Sep;9(3):200‐3. - PubMed
Pistone 2007 {published data only}
-
- Pistone T, Verdiere C‐H, Receveur M‐C, Ezzedine K, Lafon ME, Malvy D. Immunogenicity and tolerance to yellow fever vaccine in travelers living with HIV, France [Immunogenicite et tolerance du vaccin amaril chez le voyageur vivant avec le VIH, France]. Bull Epidemiol Hebdomadaire 2007;25/26:238‐40.
Pistone 2010 {published data only}
-
- Pistone T, Verdiere CH, Receveur MC, Ezzedine K, Lafon ME, Malvy D. Immunogenicity and tolerability of yellow fever vaccination in 23 French HIV‐infected patients. Curr HIV Res. 2010 Sep;8(6):461‐6. - PubMed
Receveur 2000 {published data only}
-
- Receveur MC, Thiebaut R, Vedy S, Malvy D, Mercie P, Bras ML. Yellow fever vaccination of human immunodeficiency virus‐infected patients: report of 2 cases. Clin Infect Dis. 2000 Sep;31(3):E7‐8. - PubMed
Ripoll 2008 {published data only}
-
- Ripoll C, Ponce A, Wilson MM, Sharif N, Vides JB, Armoni J, et al. Evaluation of two yellow fever vaccines for routine immunization programs in Argentina. Hum Vaccin. 2008 Mar‐Apr;4(2):121‐6. - PubMed
Roukens 2008 {published data only}
Sidibe 2012 {published data only}
-
- Sidibe M, Yactayo S, Kalle A, Sall AA, Sow S, Ndoutabe M, et al. Immunogenicity and safety of yellow fever vaccine among 115 HIV‐infected patients after a preventive immunisation campaign in Mali. Trans R Soc Trop Med Hyg. 2012 Jul;106(7):437‐44. - PubMed
Tattevin 2004 {published data only}
-
- Tattevin P, Depatureaux AG, Chapplain JM, Dupont M, Souala F, Arvieux C, et al. Yellow fever vaccine is safe and effective in HIV‐infected patients. AIDS. 2004 Mar 26;18(5):825‐7. - PubMed
Thomas 2012 {published data only}
Veit 2010 {published data only}
-
- Veit O, Hatz C, Niedrig M, Furrer H. Yellow fever vaccination in HIV‐infected patients. HIV Ther. 2010 Jan;4:17‐26.
Additional references
CDC 2010
-
- Centers for Disease Control and Prevention (CDC). Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report (MMWR) 2010;59(RR‐7):1‐27. - PubMed
GRADEpro 2011 [Computer program]
-
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro, Version 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann, 2011.
Guyatt 2008
Higgins 2008
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: Wiley‐Blackwell, 2008.
Newcastle‐Ottawa
-
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [accessed 7 January 2014].
Niedrig 1999
-
- Niedrig M, Lademann M, Emmerich P, Lafrenz M. Assessment of IgG antibodies against yellow fever virus after vaccination with 17D by different assays: neutralization test, haemagglutination inhibition test, immunofluorescence assay and ELISA. Trop Med Int Health. 1999 Dec;4(12):867‐71. - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
UNAIDS 2013
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Global Report, 2013. Available from: http://www.unaids.org/en/resources/campaigns/globalreport2013/ [accessed 7 January 2014] 2013.
WHO 2013a
-
- World Health Organization (WHO). Health Topics: Yellow Fever. Available from: http://www.who.int/topics/yellow_fever/en/ [accessed 17 December 2013].
WHO 2013b
-
- World Health Organization (WHO). Global and Alert Response (GAR): Vaccines and vaccination against yellow fever. Available from: http://www.who.int/csr/resources/publications/yellowfever/ [accessed 7 January 2013].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

