Ruthenium-catalyzed cascade C-H functionalization of phenylacetophenones
- PMID: 24453063
- PMCID: PMC4265981
- DOI: 10.1002/anie.201309114
Ruthenium-catalyzed cascade C-H functionalization of phenylacetophenones
Abstract
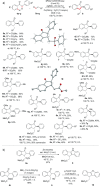
Three orthogonal cascade CH functionalization processes are described, based on ruthenium-catalyzed CH alkenylation. 1-Indanones, indeno indenes, and indeno furanones were accessed through cascade pathways by using arylacetophenones as substrates under conditions of catalytic [{Ru(p-cymene)Cl2 }2 ] and stoichiometric Cu(OAc)2 . Each transformation uses CH functionalization methods to form CC bonds sequentially, with the indeno furanone synthesis featuring a CO bond formation as the terminating step. This work demonstrates the power of ruthenium-catalyzed alkenylation as a platform reaction to develop more complex transformations, with multiple CH functionalization steps taking place in a single operation to access novel carbocyclic structures.
Keywords: C-H activation; cascade chemistry; homogeneous catalysis; oxidative coupling; ruthenium.
© 2014 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Figures




Similar articles
-
Ruthenium-Catalyzed meta-Selective C-H Bromination.Angew Chem Int Ed Engl. 2015 Sep 28;54(40):11677-80. doi: 10.1002/anie.201504390. Epub 2015 Aug 18. Angew Chem Int Ed Engl. 2015. PMID: 26288217 Free PMC article.
-
Towards ideal synthesis: alkenylation of aryl C-H bonds by a Fujiwara-Moritani reaction.Chemistry. 2014 Jan 13;20(3):634-42. doi: 10.1002/chem.201303670. Epub 2013 Dec 16. Chemistry. 2014. PMID: 24356913
-
Mechanism of Rhodium-Catalyzed C-H Functionalization: Advances in Theoretical Investigation.Acc Chem Res. 2017 Nov 21;50(11):2799-2808. doi: 10.1021/acs.accounts.7b00400. Epub 2017 Nov 7. Acc Chem Res. 2017. PMID: 29112396
-
Mechanistic view of Ru-catalyzed C-H bond activation and functionalization: computational advances.Chem Soc Rev. 2018 Oct 15;47(20):7552-7576. doi: 10.1039/c8cs00036k. Chem Soc Rev. 2018. PMID: 30182110 Review.
-
Ru-, Rh- and Ir-Catalyzed Enantioselective sp3 C-H Functionalization.Chem Asian J. 2022 Dec 14;17(24):e202200944. doi: 10.1002/asia.202200944. Epub 2022 Nov 14. Chem Asian J. 2022. PMID: 36271602 Review.
Cited by
-
Selective α-Methylation of Aryl Ketones Using Quaternary Ammonium Salts as Solid Methylating Agents.J Org Chem. 2022 Mar 18;87(6):4305-4315. doi: 10.1021/acs.joc.1c03158. Epub 2022 Mar 7. J Org Chem. 2022. PMID: 35253422 Free PMC article.
-
Ru-catalysed C-H arylation of indoles and pyrroles with boronic acids: scope and mechanistic studies.Chemistry. 2015 Mar 27;21(14):5380-6. doi: 10.1002/chem.201405931. Epub 2015 Feb 17. Chemistry. 2015. PMID: 25689052 Free PMC article.
-
Rhodium(III)-catalyzed dearomatizing (3 + 2) annulation of 2-alkenylphenols and alkynes.J Am Chem Soc. 2014 May 28;136(21):7607-10. doi: 10.1021/ja5034952. Epub 2014 May 19. J Am Chem Soc. 2014. PMID: 24820195 Free PMC article.
-
Highly chemoselective ruthenium(ii)-catalyzed direct arylation of cyclic and N,N-dialkyl benzamides with aryl silanes.Chem Sci. 2017 Apr 1;8(4):3204-3210. doi: 10.1039/c7sc00156h. Epub 2017 Feb 24. Chem Sci. 2017. PMID: 28507696 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

