Plastidial NAD-dependent malate dehydrogenase is critical for embryo development and heterotrophic metabolism in Arabidopsis
- PMID: 24453164
- PMCID: PMC3938612
- DOI: 10.1104/pp.113.233866
Plastidial NAD-dependent malate dehydrogenase is critical for embryo development and heterotrophic metabolism in Arabidopsis
Abstract
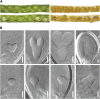
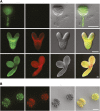
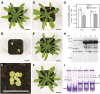
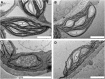
In illuminated chloroplasts, one mechanism involved in reduction-oxidation (redox) homeostasis is the malate-oxaloacetate (OAA) shuttle. Excess electrons from photosynthetic electron transport in the form of nicotinamide adenine dinucleotide phosphate, reduced are used by NADP-dependent malate dehydrogenase (MDH) to reduce OAA to malate, thus regenerating the electron acceptor NADP. NADP-MDH is a strictly redox-regulated, light-activated enzyme that is inactive in the dark. In the dark or in nonphotosynthetic tissues, the malate-OAA shuttle was proposed to be mediated by the constitutively active plastidial NAD-specific MDH isoform (pdNAD-MDH), but evidence is scarce. Here, we reveal the critical role of pdNAD-MDH in Arabidopsis (Arabidopsis thaliana) plants. A pdnad-mdh null mutation is embryo lethal. Plants with reduced pdNAD-MDH levels by means of artificial microRNA (miR-mdh-1) are viable, but dark metabolism is altered as reflected by increased nighttime malate, starch, and glutathione levels and a reduced respiration rate. In addition, miR-mdh-1 plants exhibit strong pleiotropic effects, including dwarfism, reductions in chlorophyll levels, photosynthetic rate, and daytime carbohydrate levels, and disordered chloroplast ultrastructure, particularly in developing leaves, compared with the wild type. pdNAD-MDH deficiency in miR-mdh-1 can be functionally complemented by expression of a microRNA-insensitive pdNAD-MDH but not NADP-MDH, confirming distinct roles for NAD- and NADP-linked redox homeostasis.
Figures


References
-
- Aach H, Bode H, Robinson DG, Graebe JE. (1997) ent-Kaurene synthase is located in proplastids of meristematic shoot tissues. Planta 202: 211–219
-
- Apel K, Hirt H. (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399 - PubMed
-
- Backhausen JE, Vetter S, Baalmann E, Kitzmann C, Scheibe R. (1998) NAD-dependent malate dehydrogenase and glyceraldehyde 3-phosphate dehydrogenase isoenzymes play an important role in dark metabolism of various plastid types. Planta 205: 359–366
-
- Baker A, Graham IA, Holdsworth M, Smith SM, Theodoulou FL. (2006) Chewing the fat: β-oxidation in signalling and development. Trends Plant Sci 11: 124–132 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

