Sel1L is indispensable for mammalian endoplasmic reticulum-associated degradation, endoplasmic reticulum homeostasis, and survival
- PMID: 24453213
- PMCID: PMC3918815
- DOI: 10.1073/pnas.1318114111
Sel1L is indispensable for mammalian endoplasmic reticulum-associated degradation, endoplasmic reticulum homeostasis, and survival
Erratum in
- Proc Natl Acad Sci U S A. 2014 Apr 22;111(16):6115
Abstract
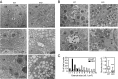
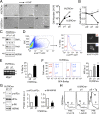
Suppressor/Enhancer of Lin-12-like (Sel1L) is an adaptor protein for the E3 ligase hydroxymethylglutaryl reductase degradation protein 1 (Hrd1) involved in endoplasmic reticulum-associated degradation (ERAD). Sel1L's physiological importance in mammalian ERAD, however, remains to be established. Here, using the inducible Sel1L knockout mouse and cell models, we show that Sel1L is indispensable for Hrd1 stability, ER homeostasis, and survival. Acute loss of Sel1L leads to premature death in adult mice within 3 wk with profound pancreatic atrophy. Contrary to current belief, our data show that mammalian Sel1L is required for Hrd1 stability and ERAD function both in vitro and in vivo. Sel1L deficiency disturbs ER homeostasis, activates ER stress, attenuates translation, and promotes cell death. Serendipitously, using a biochemical approach coupled with mass spectrometry, we found that Sel1L deficiency causes the aggregation of both small and large ribosomal subunits. Thus, Sel1L is an indispensable component of the mammalian Hrd1 ERAD complex and ER homeostasis, which is essential for protein translation, pancreatic function, and cellular and organismal survival.
Keywords: ER dilation; ERAD tuning; exocrine pancreatic insufficiency; inducible ERAD-deficient models; stress granule.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
The Integrated UPR and ERAD in Oligodendrocytes Maintain Myelin Thickness in Adults by Regulating Myelin Protein Translation.J Neurosci. 2020 Oct 21;40(43):8214-8232. doi: 10.1523/JNEUROSCI.0604-20.2020. Epub 2020 Sep 21. J Neurosci. 2020. PMID: 32958569 Free PMC article.
-
Endoplasmic reticulum associated degradation is required for maintaining endoplasmic reticulum homeostasis and viability of mature Schwann cells in adults.Glia. 2021 Feb;69(2):489-506. doi: 10.1002/glia.23910. Epub 2020 Sep 16. Glia. 2021. PMID: 32935902 Free PMC article.
-
The ER-associated degradation adaptor SEL1L is dispensable for ER homeostasis and the differentiation of spermatogenic cells.J Biol Chem. 2025 Jul;301(7):110283. doi: 10.1016/j.jbc.2025.110283. Epub 2025 May 22. J Biol Chem. 2025. PMID: 40412517 Free PMC article.
-
SEL1L-HRD1-mediated ERAD in mammals.Nat Cell Biol. 2025 Jul;27(7):1063-1073. doi: 10.1038/s41556-025-01690-1. Epub 2025 Jun 25. Nat Cell Biol. 2025. PMID: 40562846 Review.
-
Role of SEL1L in the progression of solid tumors, with a special focus on its recent therapeutic potential.Cell Biol Int. 2025 Jan;49(1):16-32. doi: 10.1002/cbin.12242. Epub 2024 Oct 4. Cell Biol Int. 2025. PMID: 39364680 Review.
Cited by
-
The HRD1-SEL1L ubiquitin ligase regulates stress granule homeostasis in couple with distinctive signaling branches of ER stress.iScience. 2024 Jun 6;27(7):110196. doi: 10.1016/j.isci.2024.110196. eCollection 2024 Jul 19. iScience. 2024. PMID: 38979013 Free PMC article.
-
Luteolin Protects Against 6-Hydoroxydopamine-Induced Cell Death via an Upregulation of HRD1 and SEL1L.Neurochem Res. 2024 Jan;49(1):117-128. doi: 10.1007/s11064-023-04019-2. Epub 2023 Aug 26. Neurochem Res. 2024. PMID: 37632637 Free PMC article.
-
Redundant and Antagonistic Roles of XTP3B and OS9 in Decoding Glycan and Non-glycan Degrons in ER-Associated Degradation.Mol Cell. 2018 May 3;70(3):516-530.e6. doi: 10.1016/j.molcel.2018.03.026. Epub 2018 Apr 26. Mol Cell. 2018. PMID: 29706535 Free PMC article.
-
Epithelial Sel1L is required for the maintenance of intestinal homeostasis.Mol Biol Cell. 2016 Feb 1;27(3):483-90. doi: 10.1091/mbc.E15-10-0724. Epub 2015 Dec 2. Mol Biol Cell. 2016. PMID: 26631554 Free PMC article.
-
Mice deficient for ERAD machinery component Sel1L develop central diabetes insipidus.J Clin Invest. 2017 Oct 2;127(10):3591-3593. doi: 10.1172/JCI96839. Epub 2017 Sep 18. J Clin Invest. 2017. PMID: 28920918 Free PMC article.
References
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. - PubMed
-
- Hampton RY, Sommer T. Finding the will and the way of ERAD substrate retrotranslocation. Curr Opin Cell Biol. 2012;24(4):460–466. - PubMed
-
- Walter P, Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

