Structure of signaling-competent neurotensin receptor 1 obtained by directed evolution in Escherichia coli
- PMID: 24453215
- PMCID: PMC3926081
- DOI: 10.1073/pnas.1317903111
Structure of signaling-competent neurotensin receptor 1 obtained by directed evolution in Escherichia coli
Abstract
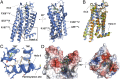
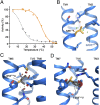
Crystallography has advanced our understanding of G protein-coupled receptors, but low expression levels and instability in solution have limited structural insights to very few selected members of this large protein family. Using neurotensin receptor 1 (NTR1) as a proof of principle, we show that two directed evolution technologies that we recently developed have the potential to overcome these problems. We purified three neurotensin-bound NTR1 variants from Escherichia coli and determined their X-ray structures at up to 2.75 Å resolution using vapor diffusion crystallization experiments. A crystallized construct was pharmacologically characterized and exhibited ligand-dependent signaling, internalization, and wild-type-like agonist and antagonist affinities. Our structures are fully consistent with all biochemically defined ligand-contacting residues, and they represent an inactive NTR1 state at the cytosolic side. They exhibit significant differences to a previously determined NTR1 structure (Protein Data Bank ID code 4GRV) in the ligand-binding pocket and by the presence of the amphipathic helix 8. A comparison of helix 8 stability determinants between NTR1 and other crystallized G protein-coupled receptors suggests that the occupancy of the canonical position of the amphipathic helix is reduced to various extents in many receptors, and we have elucidated the sequence determinants for a stable helix 8. Our analysis also provides a structural rationale for the long-known effects of C-terminal palmitoylation reactions on G protein-coupled receptor signaling, receptor maturation, and desensitization.
Keywords: detergents; membrane proteins; protein engineering; protein stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Bissette G, Nemeroff CB, Loosen PT, Prange AJ, Jr, Lipton MA. Hypothermia and intolerance to cold induced by intracisternal administration of the hypothalamic peptide neurotensin. Nature. 1976;262(5569):607–609. - PubMed
-
- Alifano M, et al. Neurotensin receptor 1 determines the outcome of non-small cell lung cancer. Clin Cancer Res. 2010;16(17):4401–4410. - PubMed
-
- Griebel G, Holsboer F. Neuropeptide receptor ligands as drugs for psychiatric diseases: The end of the beginning? Nat Rev Drug Discov. 2012;11(6):462–478. - PubMed
-
- Tanaka K, Masu M, Nakanishi S. Structure and functional expression of the cloned rat neurotensin receptor. Neuron. 1990;4(6):847–854. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

