Embryonic trophoblasts induce decidual regulatory T cell differentiation and maternal-fetal tolerance through thymic stromal lymphopoietin instructing dendritic cells
- PMID: 24453244
- PMCID: PMC3918863
- DOI: 10.4049/jimmunol.1203425
Embryonic trophoblasts induce decidual regulatory T cell differentiation and maternal-fetal tolerance through thymic stromal lymphopoietin instructing dendritic cells
Abstract
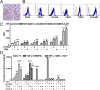
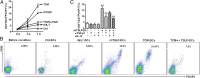
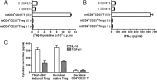
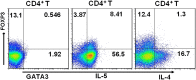
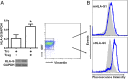
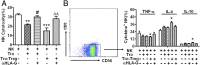
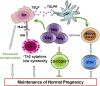
Physiological pregnancy requires the maternal immune system to recognize and tolerate embryonic Ags. Although multiple mechanisms have been proposed, it is not yet clear how the fetus evades the maternal immune system. In this article, we demonstrate that trophoblast-derived thymic stromal lymphopoietin (TSLP) instructs decidual CD11c(+) dendritic cells (dDCs)with increased costimulatory molecules; MHC class II; and Th2/3-type, but not Th1-type, cytokines. TSLP-activated dDCs induce proliferation and differentiation of decidual CD4(+)CD25(-) T cells into CD4(+)CD25(+)FOXP3(+) regulatory T cells (Tregs) through TGF-β1. TSLP-activated dDC-induced Tregs display immunosuppressive features and express Th2-type cytokines. In addition, decidual CD4(+)CD25(+)FOXP3(+) Tregs promote invasiveness and HLA-G expression of trophoblasts, resulting in preferential production of Th2 cytokines and reduced cytotoxicity in decidual CD56(bright)CD16(-) NK cells. Of interest, decreased TSLP expression and reduced numbers of Tregs were observed at the maternal-fetal interface during miscarriage. Our study identifies a novel feedback loop between embryo-derived trophoblasts and maternal decidual leukocytes, which induces a tolerogenic immune response to ensure a successful pregnancy.
Figures


References
-
- Medawar P. B. 1953. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Soc. Exp. Biol. 7: 320–328.
-
- Trowsdale J., Betz A. G. 2006. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat. Immunol. 7: 241–246. - PubMed
-
- Munoz-Suano A., Hamilton A. B., Betz A. G. 2011. Gimme shelter: the immune system during pregnancy. Immunol. Rev. 241: 20–38. - PubMed
-
- Tilburgs T., Scherjon S. A., Claas F. H. 2010. Major histocompatibility complex (MHC)-mediated immune regulation of decidual leukocytes at the fetal-maternal interface. J. Reprod. Immunol. 85: 58–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

