Dectin-2 regulates the effector phase of house dust mite-elicited pulmonary inflammation independently from its role in sensitization
- PMID: 24453247
- PMCID: PMC4024442
- DOI: 10.4049/jimmunol.1301809
Dectin-2 regulates the effector phase of house dust mite-elicited pulmonary inflammation independently from its role in sensitization
Abstract
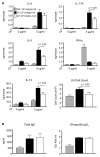
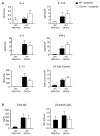
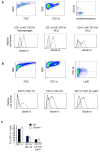
The myeloid C-type lectin receptor Dectin-2 directs the generation of Th2 and Th17 immune responses to the house dust mite Dermatophagoides farinae through the generation of cysteinyl leukotrienes and proinflammatory cytokines, respectively, but a role for Dectin-2 in effector phase responses has not been described. In this study, we demonstrate that administration of the Dectin-2 mAb solely at the time of D. farinae challenge abrogated eosinophilic and neutrophilic inflammation in the bronchoalveolar lavage fluid and Th1, Th2, and Th17 inflammation in the lung of previously sensitized mice. Furthermore, Dectin-2 null mice (Clec4n(-/-)) sensitized with the adoptive transfer of D. farinae-pulsed wild-type (WT) bone marrow-derived dendritic cells (DCs) also had less D. farinae-elicited pulmonary inflammation, supporting an effector function for Dectin-2. The protection from pulmonary inflammation seen with the Dectin-2 mAb or in Clec4n(-/-) mice was associated with little or no reduction in lung-draining lymph node cells or their cytokine production and with no reduction in serum IgE. WT and Clec4n(-/-) mice recipients, sensitized with D. farinae-pulsed WT bone marrow-derived DCs, had comparable levels of D. farinae-elicited IL-6, IL-23, TNF-α, and cysteinyl leukotrienes in the lung. By contrast, D. farinae-elicited CCL4 and CCL8 production from pulmonary CD11c(+)CD11b(+)Ly6C(+) and CD11c(+)CD11b(+)Ly6C(-)CD64(+) monocyte-derived DCs was reduced in Clec4n(-/-) recipients. Addition of CCL8 at the time of D. farinae challenge abrogated the protection from eosinophilic, neutrophilic, and Th2 pulmonary inflammation seen in Clec4n(-/-) recipients. Taken together, these results reveal that Dectin-2 regulates monocyte-derived DC function in the pulmonary microenvironment at D. farinae challenge to promote the local inflammatory response.
Figures




Similar articles
-
Phosphoinositide 3-Kinase δ Regulates Dectin-2 Signaling and the Generation of Th2 and Th17 Immunity.J Immunol. 2016 Jul 1;197(1):278-87. doi: 10.4049/jimmunol.1502485. Epub 2016 May 18. J Immunol. 2016. PMID: 27194783 Free PMC article.
-
Dectin-2 promotes house dust mite-induced T helper type 2 and type 17 cell differentiation and allergic airway inflammation in mice.Am J Respir Cell Mol Biol. 2014 Aug;51(2):201-9. doi: 10.1165/rcmb.2013-0522OC. Am J Respir Cell Mol Biol. 2014. PMID: 24588637
-
Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes.J Exp Med. 2011 Mar 14;208(3):593-604. doi: 10.1084/jem.20100793. Epub 2011 Feb 28. J Exp Med. 2011. PMID: 21357742 Free PMC article.
-
LPS-induced CD11b+Gr1(int)F4/80+ regulatory myeloid cells suppress allergen-induced airway inflammation.Int Immunopharmacol. 2011 Jul;11(7):827-32. doi: 10.1016/j.intimp.2011.01.034. Epub 2011 Feb 12. Int Immunopharmacol. 2011. PMID: 21320637 Free PMC article. Review.
-
The impact of bacteria-derived ultrafine dust particles on pulmonary diseases.Exp Mol Med. 2020 Mar;52(3):338-347. doi: 10.1038/s12276-019-0367-3. Epub 2020 Mar 17. Exp Mol Med. 2020. PMID: 32203101 Free PMC article. Review.
Cited by
-
The C-Type Lectin Receptor Dectin-2 Is a Receptor for Aspergillus fumigatus Galactomannan.mBio. 2023 Feb 28;14(1):e0318422. doi: 10.1128/mbio.03184-22. Epub 2023 Jan 4. mBio. 2023. PMID: 36598192 Free PMC article.
-
C-Type Lectin Receptor Mediated Modulation of T2 Immune Responses to Allergens.Curr Allergy Asthma Rep. 2023 Mar;23(3):141-151. doi: 10.1007/s11882-023-01067-0. Epub 2023 Feb 1. Curr Allergy Asthma Rep. 2023. PMID: 36720753 Free PMC article. Review.
-
Chemical respiratory sensitization-Current status of mechanistic understanding, knowledge gaps and possible identification methods of sensitizers.Front Toxicol. 2024 Jul 29;6:1331803. doi: 10.3389/ftox.2024.1331803. eCollection 2024. Front Toxicol. 2024. PMID: 39135743 Free PMC article. Review.
-
C-type lectin receptors in the control of T helper cell differentiation.Nat Rev Immunol. 2016 Jul;16(7):433-48. doi: 10.1038/nri.2016.55. Epub 2016 Jun 13. Nat Rev Immunol. 2016. PMID: 27291962 Review.
-
Phosphoinositide 3-Kinase δ Regulates Dectin-2 Signaling and the Generation of Th2 and Th17 Immunity.J Immunol. 2016 Jul 1;197(1):278-87. doi: 10.4049/jimmunol.1502485. Epub 2016 May 18. J Immunol. 2016. PMID: 27194783 Free PMC article.
References
-
- Huss K, Adkinson NF, Jr, Eggleston PA, Dawson C, Van Natta ML, Hamilton RG. House dust mite and cockroach exposure are strong risk factors for positive allergy skin test responses in the Childhood Asthma Management Program. J Allergy Clin Immunol. 2001;107:48–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

