Dopamine-modulated recurrent corticoefferent feedback in primary sensory cortex promotes detection of behaviorally relevant stimuli
- PMID: 24453315
- PMCID: PMC3898285
- DOI: 10.1523/JNEUROSCI.1990-13.2014
Dopamine-modulated recurrent corticoefferent feedback in primary sensory cortex promotes detection of behaviorally relevant stimuli
Abstract
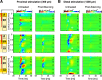
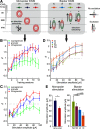
Dopaminergic neurotransmission in primary auditory cortex (AI) has been shown to be involved in learning and memory functions. Moreover, dopaminergic projections and D1/D5 receptor distributions display a layer-dependent organization, suggesting specific functions in the cortical circuitry. However, the circuit effects of dopaminergic neurotransmission in sensory cortex and their possible roles in perception, learning, and memory are largely unknown. Here, we investigated layer-specific circuit effects of dopaminergic neuromodulation using current source density (CSD) analysis in AI of Mongolian gerbils. Pharmacological stimulation of D1/D5 receptors increased auditory-evoked synaptic currents in infragranular layers, prolonging local thalamocortical input via positive feedback between infragranular output and granular input. Subsequently, dopamine promoted sustained cortical activation by prolonged recruitment of long-range corticocortical networks. A detailed circuit analysis combining layer-specific intracortical microstimulation (ICMS), CSD analysis, and pharmacological cortical silencing revealed that cross-laminar feedback enhanced by dopamine relied on a positive, fast-acting recurrent corticoefferent loop, most likely relayed via local thalamic circuits. Behavioral signal detection analysis further showed that activation of corticoefferent output by infragranular ICMS, which mimicked auditory activation under dopaminergic influence, was most effective in eliciting a behaviorally detectable signal. Our results show that D1/D5-mediated dopaminergic modulation in sensory cortex regulates positive recurrent corticoefferent feedback, which enhances states of high, persistent activity in sensory cortex evoked by behaviorally relevant stimuli. In boosting horizontal network interactions, this potentially promotes the readout of task-related information from cortical synapses and improves behavioral stimulus detection.
Keywords: auditory cortex; behavior; corticoefferent recurrence; dopamine; perception.
Figures





Similar articles
-
Laser-Induced Apoptosis of Corticothalamic Neurons in Layer VI of Auditory Cortex Impact on Cortical Frequency Processing.Front Neural Circuits. 2021 Jul 12;15:659280. doi: 10.3389/fncir.2021.659280. eCollection 2021. Front Neural Circuits. 2021. PMID: 34322001 Free PMC article.
-
Dopaminergic neuromodulation of high gamma stimulus phase-locking in gerbil primary auditory cortex mediated by D1/D5-receptors.Eur J Neurosci. 2020 Mar;51(5):1315-1327. doi: 10.1111/ejn.13898. Epub 2018 Mar 31. Eur J Neurosci. 2020. PMID: 29514417
-
Optogenetic stimulation of the VTA modulates a frequency-specific gain of thalamocortical inputs in infragranular layers of the auditory cortex.Sci Rep. 2019 Dec 31;9(1):20385. doi: 10.1038/s41598-019-56926-6. Sci Rep. 2019. PMID: 31892726 Free PMC article.
-
Presynaptic gating of postsynaptic synaptic plasticity: a plasticity filter in the adult auditory cortex.Neuroscientist. 2013 Oct;19(5):465-78. doi: 10.1177/1073858413482983. Epub 2013 Apr 4. Neuroscientist. 2013. PMID: 23558179 Free PMC article. Review.
-
Dopaminergic impact on local and global cortical circuit processing during learning.Behav Brain Res. 2016 Feb 15;299:32-41. doi: 10.1016/j.bbr.2015.11.016. Epub 2015 Nov 30. Behav Brain Res. 2016. PMID: 26608540 Review.
Cited by
-
β-adrenergic modulation of discrimination learning and memory in the auditory cortex.Eur J Neurosci. 2019 Oct;50(7):3141-3163. doi: 10.1111/ejn.14480. Epub 2019 Jul 1. Eur J Neurosci. 2019. PMID: 31162753 Free PMC article.
-
When and How Does the Auditory Cortex Influence Subcortical Auditory Structures? New Insights About the Roles of Descending Cortical Projections.Front Neurosci. 2021 Aug 3;15:690223. doi: 10.3389/fnins.2021.690223. eCollection 2021. Front Neurosci. 2021. PMID: 34413722 Free PMC article. Review.
-
Associations between visual perception accuracy and confidence in a dopaminergic manipulation study.Front Psychol. 2015 Apr 16;6:414. doi: 10.3389/fpsyg.2015.00414. eCollection 2015. Front Psychol. 2015. PMID: 25932015 Free PMC article.
-
Complementary control of sensory adaptation by two types of cortical interneurons.Elife. 2015 Oct 13;4:e09868. doi: 10.7554/eLife.09868. Elife. 2015. PMID: 26460542 Free PMC article.
-
Spectral breadth and laminar distribution of thalamocortical inputs to A1.J Neurophysiol. 2016 Apr;115(4):2083-94. doi: 10.1152/jn.00887.2015. Epub 2016 Feb 17. J Neurophysiol. 2016. PMID: 26888102 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
