p140Cap regulates memory and synaptic plasticity through Src-mediated and citron-N-mediated actin reorganization
- PMID: 24453341
- PMCID: PMC6705312
- DOI: 10.1523/JNEUROSCI.2341-13.2014
p140Cap regulates memory and synaptic plasticity through Src-mediated and citron-N-mediated actin reorganization
Abstract
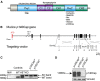
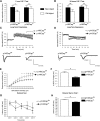
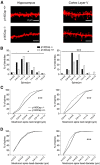
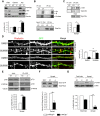
A major challenge in the neuroscience field is the identification of molecules and pathways that control synaptic plasticity and memory. Dendritic spines play a pivotal role in these processes, as the major sites of excitatory synapses in neuronal communication. Previous studies have shown that the scaffold protein p140Cap localizes into dendritic spines and that its knockdown negatively modulates spine shape in culture. However, so far, there is no information on its in vivo relevance. By using a knock-out mouse model, we here demonstrate that p140Cap is a key element for both learning and synaptic plasticity. Indeed, p140Cap(-/-) mice are impaired in object recognition test, as well as in LTP and in LTD measurements. The in vivo effects of p140Cap loss are presumably attenuated by noncell-autonomous events, since primary neurons obtained from p140Cap(-/-) mice show a strong reduction in number of mushroom spines and abnormal organization of synapse-associated F-actin. These phenotypes are most likely caused by a local reduction of the inhibitory control of RhoA and of cortactin toward the actin-depolymerizing factor cofilin. These events can be controlled by p140Cap through its capability to directly inhibit the activation of Src kinase and by its binding to the scaffold protein Citron-N. Altogether, our results provide new insight into how protein associated with dynamic microtubules may regulate spine actin organization through interaction with postsynaptic density components.
Keywords: Cit-N; Rho GTPase; Src; actin cytoskeleton; p140Cap; synapses.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
