Efficacy and mechanism of a glycoside compound inhibiting abnormal prion protein formation in prion-infected cells: implications of interferon and phosphodiesterase 4D-interacting protein
- PMID: 24453367
- PMCID: PMC3993762
- DOI: 10.1128/JVI.03775-13
Efficacy and mechanism of a glycoside compound inhibiting abnormal prion protein formation in prion-infected cells: implications of interferon and phosphodiesterase 4D-interacting protein
Abstract
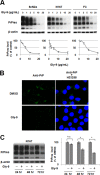
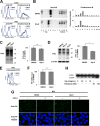
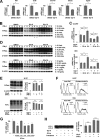
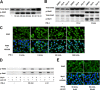
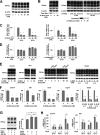
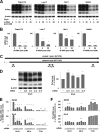
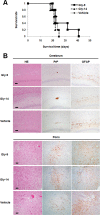
A new type of antiprion compound, Gly-9, was found to inhibit abnormal prion protein formation in prion-infected neuroblastoma cells, in a prion strain-independent manner, when the cells were treated for more than 1 day. It reduced the intracellular prion protein level and significantly modified mRNA expression levels of genes of two types: interferon-stimulated genes were downregulated after more than 2 days of treatment, and the phosphodiesterase 4D-interacting protein gene, a gene involved in microtubule growth, was upregulated after more than 1 day of treatment. A supplement of interferon given to the cells partly restored the abnormal prion protein level but did not alter the normal prion protein level. This interferon action was independent of the Janus activated kinase-signal transducer and activator of transcription signaling pathway. Therefore, the changes in interferon-stimulated genes might be a secondary effect of Gly-9 treatment. However, gene knockdown of phosphodiesterase 4D-interacting protein restored or increased both the abnormal prion protein level and the normal prion protein level, without transcriptional alteration of the prion protein gene. It also altered the localization of abnormal prion protein accumulation in the cells, indicating that phosphodiesterase 4D-interacting protein might affect prion protein levels by altering the trafficking of prion protein-containing structures. Interferon and phosphodiesterase 4D-interacting protein had no direct mutual link, demonstrating that they regulate abnormal prion protein levels independently. Although the in vivo efficacy of Gly-9 was limited, the findings for Gly-9 provide insights into the regulation of abnormal prion protein in cells and suggest new targets for antiprion compounds.
Importance: This report describes our study of the efficacy and potential mechanism underlying the antiprion action of a new antiprion compound with a glycoside structure in prion-infected cells, as well as the efficacy of the compound in prion-infected animals. The study revealed involvements of two factors in the compound's mechanism of action: interferon and a microtubule nucleation activator, phosphodiesterase 4D-interacting protein. In particular, phosphodiesterase 4D-interacting protein was suggested to be important in regulating the trafficking or fusion of prion protein-containing vesicles or structures in cells. The findings of the study are expected to be useful not only for the elucidation of cellular regulatory mechanisms of prion protein but also for the implication of new targets for therapeutic development.
Figures


References
-
- Caughey B. 1991. In vitro expression and biosynthesis of prion protein. Curr. Top. Microbiol. Immunol. 172:93–107 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

