TNF and TNF receptor superfamily members in HIV infection: new cellular targets for therapy?
- PMID: 24453421
- PMCID: PMC3880767
- DOI: 10.1155/2013/484378
TNF and TNF receptor superfamily members in HIV infection: new cellular targets for therapy?
Abstract
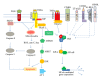
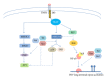
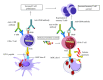
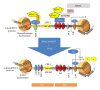
Tumor necrosis factor (TNF) and TNF receptors (TNFR) superfamily members are engaged in diverse cellular phenomena such as cellular proliferation, morphogenesis, apoptosis, inflammation, and immune regulation. Their role in regulating viral infections has been well documented. Viruses have evolved with numerous strategies to interfere with TNF-mediated signaling indicating the importance of TNF and TNFR superfamily in viral pathogenesis. Recent research reports suggest that TNF and TNFRs play an important role in the pathogenesis of HIV. TNFR signaling modulates HIV replication and HIV proteins interfere with TNF/TNFR pathways. Since immune activation and inflammation are the hallmark of HIV infection, the use of TNF inhibitors can have significant impact on HIV disease progression. In this review, we will describe how HIV infection is modulated by signaling mediated through members of TNF and TNFR superfamily and in turn how these latter could be targeted by HIV proteins. Finally, we will discuss the emerging therapeutics options based on modulation of TNF activity that could ultimately lead to the cure of HIV-infected patients.
Figures
Similar articles
-
Targeting TNF and TNF Receptor Pathway in HIV-1 Infection: from Immune Activation to Viral Reservoirs.Viruses. 2017 Mar 30;9(4):64. doi: 10.3390/v9040064. Viruses. 2017. PMID: 28358311 Free PMC article. Review.
-
Tumor necrosis factor receptor superfamily members and their ligands.Curr Opin Immunol. 1994 Jun;6(3):407-13. doi: 10.1016/0952-7915(94)90119-8. Curr Opin Immunol. 1994. PMID: 7917108 Review.
-
Cooperation of TNF family members CD40 ligand, receptor activator of NF-kappa B ligand, and TNF-alpha in the activation of dendritic cells and the expansion of viral specific CD8+ T cell memory responses in HIV-1-infected and HIV-1-uninfected individuals.J Immunol. 2003 Feb 15;170(4):1797-805. doi: 10.4049/jimmunol.170.4.1797. J Immunol. 2003. PMID: 12574344
-
The effects of malignant transformation on susceptibility of human urothelial cells to CD40-mediated apoptosis.J Natl Cancer Inst. 2002 Sep 18;94(18):1381-95. doi: 10.1093/jnci/94.18.1381. J Natl Cancer Inst. 2002. PMID: 12237284
-
Structural and biological features of the TNF receptor and TNF ligand superfamilies: interactive signals in the pathobiology of Hodgkin's disease.Ann Oncol. 1996;7 Suppl 4:19-26. doi: 10.1093/annonc/7.suppl_4.s19. Ann Oncol. 1996. PMID: 8836404 Review.
Cited by
-
HIV-1 latency in monocytes/macrophages.Viruses. 2014 Apr 22;6(4):1837-60. doi: 10.3390/v6041837. Viruses. 2014. PMID: 24759213 Free PMC article. Review.
-
The transcriptome of HIV-1 infected intestinal CD4+ T cells exposed to enteric bacteria.PLoS Pathog. 2017 Feb 27;13(2):e1006226. doi: 10.1371/journal.ppat.1006226. eCollection 2017 Feb. PLoS Pathog. 2017. PMID: 28241075 Free PMC article.
-
NK Cells in HIV-1 Infection: From Basic Science to Vaccine Strategies.Front Immunol. 2018 Oct 17;9:2290. doi: 10.3389/fimmu.2018.02290. eCollection 2018. Front Immunol. 2018. PMID: 30386329 Free PMC article. Review.
-
Management of psoriatic patients in biologic treatment associated with infectious comorbidities.Postepy Dermatol Alergol. 2020 Jun;37(3):417-421. doi: 10.5114/ada.2020.96155. Epub 2020 Jul 16. Postepy Dermatol Alergol. 2020. PMID: 32792886 Free PMC article.
-
Inflammatory biomarker levels over 48 weeks with dual vs triple lopinavir/ritonavir-based therapy: Substudy of a randomized trial.PLoS One. 2019 Sep 6;14(9):e0221653. doi: 10.1371/journal.pone.0221653. eCollection 2019. PLoS One. 2019. PMID: 31490959 Free PMC article.
References
-
- Beutler B, Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986;320(6063):584–588. - PubMed
-
- Bradley JR. TNF-mediated inflammatory disease. Journal of Pathology. 2008;214(2):149–160. - PubMed
-
- Moelants EA, Mortier A, van Damme J, Proost P. Regulation of TNF-alpha with a focus on rheumatoid arthritis. Immunology & Cell Biology. 2013;91(6):393–401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

