Treatment discontinuation and disease progression with injectable disease-modifying therapies: findings from the north american research committee on multiple sclerosis database
- PMID: 24453783
- PMCID: PMC3883017
- DOI: 10.7224/1537-2073.2012-034
Treatment discontinuation and disease progression with injectable disease-modifying therapies: findings from the north american research committee on multiple sclerosis database
Abstract
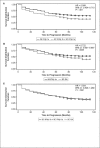
Injectable first-line disease-modifying therapies (DMTs) for multiple sclerosis (MS) are generally prescribed for continuous use. Accordingly, the various factors that influence patient persistence with treatment and that can lead some patients to switch medications or discontinue treatment may affect clinical outcomes. Using data from the North American Research Committee on Multiple Sclerosis (NARCOMS) database, this study evaluated participants' reasons for discontinuation of injectable DMTs as well as the relationship between staying on therapy and sustained patient-reported disease progression and annualized relapse rates. Participants selected their reason(s) for discontinuation from among 16 possible options covering the categories of efficacy, safety, tolerability, and burden, with multiple responses permitted. Both unadjusted data and data adjusted for baseline age, disease duration, disability, and sex were evaluated. Discontinuation profiles varied among DMTs. Participants on intramuscular interferon beta-1a (IM IFNβ-1a) and glatiramer acetate (GA) reported the fewest discontinuations based on safety concerns, although GA was associated with reports of higher burden and lower efficacy than other therapies. Difficulties with tolerability were more often reported as a reason for discontinuing subcutaneous (SC) IFNβ-1a than as a reason for discontinuing IM IFNβ-1a, GA, or SC IFNβ-1b. In the persistent therapy cohort, less patient-reported disability progression was reported with IM IFNβ-1a treatment than with SC IFNβ-1a, IFNβ-1b, or GA. These findings have relevance to clinical decision making and medication compliance in MS patient care.
Figures
References
-
- Stys PK. Multiple sclerosis: autoimmune disease or autoimmune reaction? Can J Neurol Sci. 2010;37(suppl 2):S16–S23. - PubMed
-
- Fox RJ, Arnold DL. Seeing injectable MS therapies differently: they are more similar than different. Neurology. 2009;72:1972–1973. - PubMed
-
- Tremlett HL, Oger J. Interrupted therapy: stopping and switching of the beta-interferons prescribed for MS. Neurology. 2003;61:551–554. - PubMed
-
- Tremlett H, Van der Mei I, Pittas F et al. Adherence to the immuno-modulatory drugs for multiple sclerosis: contrasting factors affect stopping drug and missing doses. Pharmacoepidemiol Drug Saf. 2008;17:565–576. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
