Tissue engineering of urinary bladder and urethra: advances from bench to patients
- PMID: 24453796
- PMCID: PMC3886608
- DOI: 10.1155/2013/154564
Tissue engineering of urinary bladder and urethra: advances from bench to patients
Abstract
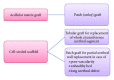
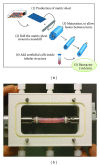
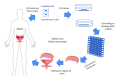
Urinary tract is subjected to many varieties of pathologies since birth including congenital anomalies, trauma, inflammatory lesions, and malignancy. These diseases necessitate the replacement of involved organs and tissues. Shortage of organ donation, problems of immunosuppression, and complications associated with the use of nonnative tissues have urged clinicians and scientists to investigate new therapies, namely, tissue engineering. Tissue engineering follows principles of cell transplantation, materials science, and engineering. Epithelial and muscle cells can be harvested and used for reconstruction of the engineered grafts. These cells must be delivered in a well-organized and differentiated condition because water-seal epithelium and well-oriented muscle layer are needed for proper function of the substitute tissues. Synthetic or natural scaffolds have been used for engineering lower urinary tract. Harnessing autologous cells to produce their own matrix and form scaffolds is a new strategy for engineering bladder and urethra. This self-assembly technique avoids the biosafety and immunological reactions related to the use of biodegradable scaffolds. Autologous equivalents have already been produced for pigs (bladder) and human (urethra and bladder). The purpose of this paper is to present a review for the existing methods of engineering bladder and urethra and to point toward perspectives for their replacement.
Figures


References
-
- Chung BI, Sommer G, Brooks JD. Anatomy of the lower urinary tract and male genitalia. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 10th edition. chapter 2. Philadelphia, Pa, USA: Saunders; 2012. pp. 33–70.
-
- Burbige KA, Hensle TW. The complications of urinary tract reconstruction. Journal of Urology. 1986;136(1, part 2):292–297. - PubMed
-
- Vemulakonda VM, Lendvay TS, Shnorhavorian M, et al. Metastatic adenocarcinoma after augmentation gastrocystoplasty. Journal of Urology. 2008;179(3):1094–1097. - PubMed
-
- McDougal WS. Metabolic complications of urinary intestinal diversion. Journal of Urology. 1992;147(5):1199–1208. - PubMed
-
- Mundy AR, Nurse DE. Calcium balance, growth and skeletal mineralisation in patients with cystoplasties. The British Journal of Urology. 1992;69(3):257–259. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

