Fabrication and evaluation of porous beta-tricalcium phosphate/hydroxyapatite (60/40) composite as a bone graft extender using rat calvarial bone defect model
- PMID: 24453864
- PMCID: PMC3878745
- DOI: 10.1155/2013/481789
Fabrication and evaluation of porous beta-tricalcium phosphate/hydroxyapatite (60/40) composite as a bone graft extender using rat calvarial bone defect model
Abstract
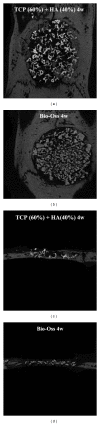
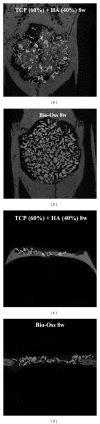
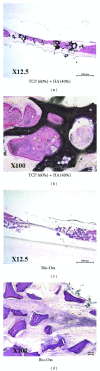
Beta-tricalcium phosphate ( β -TCP) and hydroxyapatite (HA) are widely used as bone graft extenders due to their osteoconductivity and high bioactivity. This study aims to evaluate the possibility of using porous substrate with composite ceramics ( β -TCP: HA = 60% : 40%, 60TCP40HA) as a bone graft extender and comparing it with Bio-Oss. Interconnectivity and macroporosity of β -TCP porous substrate were 99.9% and 83%, respectively, and the macro-porosity of packed granule after crushing was 69%. Calvarial defect model with 8 mm diameter was generated with male Sprague-Dawley rats and 60TCP40HA was implanted. Bio-Oss was implanted for a control group and micro-CT and histology were performed at 4 and 8 weeks after implantation. The 60TCP40HA group showed better new bone formation than the Bio-Oss group and the bone formation at central area of bone defect was increased at 8 weeks in micro-CT and histology. The percent bone volume and trabecular number of the 60TCP40HA group were significantly higher than those of Bio-Oss group. This study confirms the usefulness of the porous 60TCP40HA composite as a bone graft extender by showing increased new bone formation in the calvarial defect model and improved bone formation both quantitatively and qualitatively when compared to Bio-Oss.
Figures





References
-
- Boden SD. Overview of the biology of lumbar spine fusion and principles for selecting a bone graft substitute. Spine. 2002;27(16, supplement 1):S26–S31. - PubMed
-
- Lee JH, Hwang C-J, Song B-W, Koo K-H, Chang B-S, Lee C-K. A prospective consecutive study of instrumented posterolateral lumbar fusion using synthetic hydroxyapatite (Bongros-HA) as a bone graft extender. Journal of Biomedical Materials Research: Part A. 2009;90(3):804–810. - PubMed
-
- Boden SD, Martin GJ, Jr., Morone M, Ugbo JL, Titus L, Hutton WC. The use of coralline hydroxyapatite with bone marrow, autogenous bone graft, or osteoinductive bone protein extract for posterolateral lumbar spine fusion. Spine. 1999;24(4):320–327. - PubMed
-
- Shires R, Kessler GM. The absorption of tricalcium phosphate and its acute metabolic effects. Calcified Tissue International. 1990;47(3):142–144. - PubMed
-
- Kondo N, Ogose A, Tokunaga K, et al. Bone formation and resorption of highly purified β-tricalcium phosphate in the rat femoral condyle. Biomaterials. 2005;26(28):5600–5608. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

