KSHV 2.0: a comprehensive annotation of the Kaposi's sarcoma-associated herpesvirus genome using next-generation sequencing reveals novel genomic and functional features
- PMID: 24453964
- PMCID: PMC3894221
- DOI: 10.1371/journal.ppat.1003847
KSHV 2.0: a comprehensive annotation of the Kaposi's sarcoma-associated herpesvirus genome using next-generation sequencing reveals novel genomic and functional features
Abstract
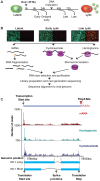
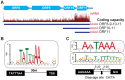
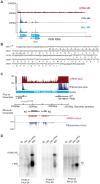
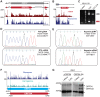
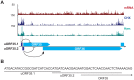
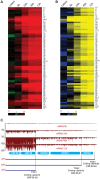
Productive herpesvirus infection requires a profound, time-controlled remodeling of the viral transcriptome and proteome. To gain insights into the genomic architecture and gene expression control in Kaposi's sarcoma-associated herpesvirus (KSHV), we performed a systematic genome-wide survey of viral transcriptional and translational activity throughout the lytic cycle. Using mRNA-sequencing and ribosome profiling, we found that transcripts encoding lytic genes are promptly bound by ribosomes upon lytic reactivation, suggesting their regulation is mainly transcriptional. Our approach also uncovered new genomic features such as ribosome occupancy of viral non-coding RNAs, numerous upstream and small open reading frames (ORFs), and unusual strategies to expand the virus coding repertoire that include alternative splicing, dynamic viral mRNA editing, and the use of alternative translation initiation codons. Furthermore, we provide a refined and expanded annotation of transcription start sites, polyadenylation sites, splice junctions, and initiation/termination codons of known and new viral features in the KSHV genomic space which we have termed KSHV 2.0. Our results represent a comprehensive genome-scale image of gene regulation during lytic KSHV infection that substantially expands our understanding of the genomic architecture and coding capacity of the virus.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Cesarman E, Knowles DM (1999) The role of Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in lymphoproliferative diseases. Seminars in cancer biology 9: 165–174. - PubMed
-
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, et al. (1994) Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science (New York, NY) 1865–1869. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

