Malaria-induced NLRP12/NLRP3-dependent caspase-1 activation mediates inflammation and hypersensitivity to bacterial superinfection
- PMID: 24453977
- PMCID: PMC3894209
- DOI: 10.1371/journal.ppat.1003885
Malaria-induced NLRP12/NLRP3-dependent caspase-1 activation mediates inflammation and hypersensitivity to bacterial superinfection
Erratum in
- PLoS Pathog. 2014 Jun;10(6):e1004258
Abstract
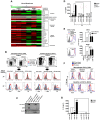
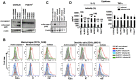
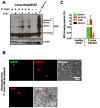
Cyclic paroxysm and high fever are hallmarks of malaria and are associated with high levels of pyrogenic cytokines, including IL-1β. In this report, we describe a signature for the expression of inflammasome-related genes and caspase-1 activation in malaria. Indeed, when we infected mice, Plasmodium infection was sufficient to promote MyD88-mediated caspase-1 activation, dependent on IFN-γ-priming and the expression of inflammasome components ASC, P2X7R, NLRP3 and/or NLRP12. Pro-IL-1β expression required a second stimulation with LPS and was also dependent on IFN-γ-priming and functional TNFR1. As a consequence of Plasmodium-induced caspase-1 activation, mice produced extremely high levels of IL-1β upon a second microbial stimulus, and became hypersensitive to septic shock. Therapeutic intervention with IL-1 receptor antagonist prevented bacterial-induced lethality in rodents. Similar to mice, we observed a significantly increased frequency of circulating CD14(+)CD16(-)Caspase-1(+) and CD14(dim)CD16(+)Caspase-1(+) monocytes in peripheral blood mononuclear cells from febrile malaria patients. These cells readily produced large amounts of IL-1β after stimulation with LPS. Furthermore, we observed the presence of inflammasome complexes in monocytes from malaria patients containing either NLRP3 or NLRP12 pyroptosomes. We conclude that NLRP12/NLRP3-dependent activation of caspase-1 is likely to be a key event in mediating systemic production of IL-1β and hypersensitivity to secondary bacterial infection during malaria.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, et al. (2012) Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379: 413–431. - PubMed
-
- Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, et al. (2009) Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis 9: 555–566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

