Glutamate utilization couples oxidative stress defense and the tricarboxylic acid cycle in Francisella phagosomal escape
- PMID: 24453979
- PMCID: PMC3894225
- DOI: 10.1371/journal.ppat.1003893
Glutamate utilization couples oxidative stress defense and the tricarboxylic acid cycle in Francisella phagosomal escape
Abstract
Intracellular bacterial pathogens have developed a variety of strategies to avoid degradation by the host innate immune defense mechanisms triggered upon phagocytocis. Upon infection of mammalian host cells, the intracellular pathogen Francisella replicates exclusively in the cytosolic compartment. Hence, its ability to escape rapidly from the phagosomal compartment is critical for its pathogenicity. Here, we show for the first time that a glutamate transporter of Francisella (here designated GadC) is critical for oxidative stress defense in the phagosome, thus impairing intra-macrophage multiplication and virulence in the mouse model. The gadC mutant failed to efficiently neutralize the production of reactive oxygen species. Remarkably, virulence of the gadC mutant was partially restored in mice defective in NADPH oxidase activity. The data presented highlight links between glutamate uptake, oxidative stress defense, the tricarboxylic acid cycle and phagosomal escape. This is the first report establishing the role of an amino acid transporter in the early stage of the Francisella intracellular lifecycle.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

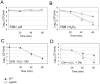
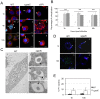
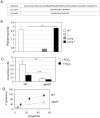
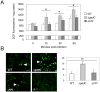
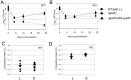
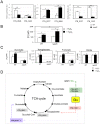
 means the ratio (H2O2-treated/non-treated) is lower in the mutant strain than in the wild-type strain; ↗ the ratio (H2O2-treated/non-treated) is higher in the mutant strain than in the wild-type strain. In the absence of external glutamate (e.g. in standard chemically defined medium), the pool of glutamate present in the bacterial cytoplasm may be synthesized either from oxoglutarate, glutamine, GSH or even proline (according to KEGG metabolic pathways).
means the ratio (H2O2-treated/non-treated) is lower in the mutant strain than in the wild-type strain; ↗ the ratio (H2O2-treated/non-treated) is higher in the mutant strain than in the wild-type strain. In the absence of external glutamate (e.g. in standard chemically defined medium), the pool of glutamate present in the bacterial cytoplasm may be synthesized either from oxoglutarate, glutamine, GSH or even proline (according to KEGG metabolic pathways).References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

