Bioelectric signaling regulates size in zebrafish fins
- PMID: 24453984
- PMCID: PMC3894163
- DOI: 10.1371/journal.pgen.1004080
Bioelectric signaling regulates size in zebrafish fins
Abstract
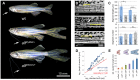
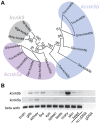
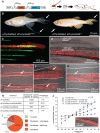
The scaling relationship between the size of an appendage or organ and that of the body as a whole is tightly regulated during animal development. If a structure grows at a different rate than the rest of the body, this process is termed allometric growth. The zebrafish another longfin (alf) mutant shows allometric growth resulting in proportionally enlarged fins and barbels. We took advantage of this mutant to study the regulation of size in vertebrates. Here, we show that alf mutants carry gain-of-function mutations in kcnk5b, a gene encoding a two-pore domain potassium (K(+)) channel. Electrophysiological analysis in Xenopus oocytes reveals that these mutations cause an increase in K(+) conductance of the channel and lead to hyperpolarization of the cell. Further, somatic transgenesis experiments indicate that kcnk5b acts locally within the mesenchyme of fins and barbels to specify appendage size. Finally, we show that the channel requires the ability to conduct K(+) ions to increase the size of these structures. Our results provide evidence for a role of bioelectric signaling through K(+) channels in the regulation of allometric scaling and coordination of growth in the zebrafish.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Huxley JS, Teissier G (1936) Terminology of relative growth. Nature 137: 780–781.
-
- Gould SJ (1966) Allometry and size in ontogeny and phylogeny. Biol Rev Camb Philos Soc 41: 587–640. - PubMed
-
- Metcalf D (1963) The autonomous behaviour of normal thymus grafts. Aust J Exp Biol Med Sci 41: SUPPL437–447. - PubMed
-
- Metcalf D (1964) Restricted growth capacity of multiple spleen grafts. Transplantation 2: 387–392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

