Is non-homologous end-joining really an inherently error-prone process?
- PMID: 24453986
- PMCID: PMC3894167
- DOI: 10.1371/journal.pgen.1004086
Is non-homologous end-joining really an inherently error-prone process?
Abstract
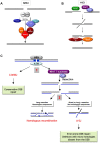
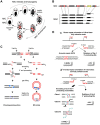
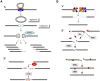
DNA double-strand breaks (DSBs) are harmful lesions leading to genomic instability or diversity. Non-homologous end-joining (NHEJ) is a prominent DSB repair pathway, which has long been considered to be error-prone. However, recent data have pointed to the intrinsic precision of NHEJ. Three reasons can account for the apparent fallibility of NHEJ: 1) the existence of a highly error-prone alternative end-joining process; 2) the adaptability of canonical C-NHEJ (Ku- and Xrcc4/ligase IV-dependent) to imperfect complementary ends; and 3) the requirement to first process chemically incompatible DNA ends that cannot be ligated directly. Thus, C-NHEJ is conservative but adaptable, and the accuracy of the repair is dictated by the structure of the DNA ends rather than by the C-NHEJ machinery. We present data from different organisms that describe the conservative/versatile properties of C-NHEJ. The advantages of the adaptability/versatility of C-NHEJ are discussed for the development of the immune repertoire and the resistance to ionizing radiation, especially at low doses, and for targeted genome manipulation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Guirouilh-Barbat J, Huck S, Bertrand P, Pirzio L, Desmaze C, et al. (2004) Impact of the KU80 pathway on NHEJ-induced genome rearrangements in mammalian cells. Mol Cell 14: 611–623. - PubMed
-
- Audebert M, Salles B, Calsou P (2004) Involvement of poly(ADP-ribose) polymerase-1 and XRCC1/DNA ligase III in an alternative route for DNA double-strand breaks rejoining. J Biol Chem 279: 55117–55126. - PubMed
-
- Corneo B, Wendland RL, Deriano L, Cui X, Klein IA, et al. (2007) Rag mutations reveal robust alternative end joining. Nature 449: 483–486. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

