Genomic confirmation of hybridisation and recent inbreeding in a vector-isolated Leishmania population
- PMID: 24453988
- PMCID: PMC3894156
- DOI: 10.1371/journal.pgen.1004092
Genomic confirmation of hybridisation and recent inbreeding in a vector-isolated Leishmania population
Abstract
Although asexual reproduction via clonal propagation has been proposed as the principal reproductive mechanism across parasitic protozoa of the Leishmania genus, sexual recombination has long been suspected, based on hybrid marker profiles detected in field isolates from different geographical locations. The recent experimental demonstration of a sexual cycle in Leishmania within sand flies has confirmed the occurrence of hybridisation, but knowledge of the parasite life cycle in the wild still remains limited. Here, we use whole genome sequencing to investigate the frequency of sexual reproduction in Leishmania, by sequencing the genomes of 11 Leishmania infantum isolates from sand flies and 1 patient isolate in a focus of cutaneous leishmaniasis in the Çukurova province of southeast Turkey. This is the first genome-wide examination of a vector-isolated population of Leishmania parasites. A genome-wide pattern of patchy heterozygosity and SNP density was observed both within individual strains and across the whole group. Comparisons with other Leishmania donovani complex genome sequences suggest that these isolates are derived from a single cross of two diverse strains with subsequent recombination within the population. This interpretation is supported by a statistical model of the genomic variability for each strain compared to the L. infantum reference genome strain as well as genome-wide scans for recombination within the population. Further analysis of these heterozygous blocks indicates that the two parents were phylogenetically distinct. Patterns of linkage disequilibrium indicate that this population reproduced primarily clonally following the original hybridisation event, but that some recombination also occurred. This observation allowed us to estimate the relative rates of sexual and asexual reproduction within this population, to our knowledge the first quantitative estimate of these events during the Leishmania life cycle.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



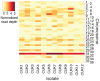
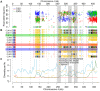
Similar articles
-
Natural hybrid of Leishmania infantum/L. donovani: development in Phlebotomus tobbi, P. perniciosus and Lutzomyia longipalpis and comparison with non-hybrid strains differing in tissue tropism.Parasit Vectors. 2015 Nov 25;8:605. doi: 10.1186/s13071-015-1217-3. Parasit Vectors. 2015. PMID: 26608249 Free PMC article.
-
Investigation of Phlebotominae (Diptera: Psychodidae) Fauna, Seasonal Dynamics, and Natural Leishmania spp. Infection in Muğla, Southwest of Turkey.Acta Trop. 2021 Apr;216:105827. doi: 10.1016/j.actatropica.2021.105827. Epub 2021 Jan 9. Acta Trop. 2021. PMID: 33428877
-
Extreme inbreeding in Leishmania braziliensis.Proc Natl Acad Sci U S A. 2009 Jun 23;106(25):10224-9. doi: 10.1073/pnas.0904420106. Epub 2009 Jun 4. Proc Natl Acad Sci U S A. 2009. PMID: 19497885 Free PMC article.
-
Reproduction in Leishmania: A focus on genetic exchange.Infect Genet Evol. 2017 Jun;50:128-132. doi: 10.1016/j.meegid.2016.10.013. Epub 2016 Oct 19. Infect Genet Evol. 2017. PMID: 27769896 Review.
-
Leishmania and the leishmaniases: a parasite genetic update and advances in taxonomy, epidemiology and pathogenicity in humans.Adv Parasitol. 2007;64:1-109. doi: 10.1016/S0065-308X(06)64001-3. Adv Parasitol. 2007. PMID: 17499100 Review.
Cited by
-
Complex Investigation of a Pediatric Haematological Case: Haemophagocytic Syndrome Associated with Visceral Leishmaniasis and Epstein⁻Barr (EBV) Co-Infection.Int J Environ Res Public Health. 2018 Nov 27;15(12):2672. doi: 10.3390/ijerph15122672. Int J Environ Res Public Health. 2018. PMID: 30486459 Free PMC article.
-
PCR-RFLP analyses of Leishmania species causing cutaneous and mucocutaneous leishmaniasis revealed distribution of genetically complex strains with hybrid and mito-nuclear discordance in Ecuador.PLoS Negl Trop Dis. 2019 May 6;13(5):e0007403. doi: 10.1371/journal.pntd.0007403. eCollection 2019 May. PLoS Negl Trop Dis. 2019. PMID: 31059516 Free PMC article.
-
A novel strain of Leishmania braziliensis harbors not a toti- but a bunyavirus.PLoS Negl Trop Dis. 2024 Dec 27;18(12):e0012767. doi: 10.1371/journal.pntd.0012767. eCollection 2024 Dec. PLoS Negl Trop Dis. 2024. PMID: 39729426 Free PMC article.
-
Population Structure of Leishmania tropica Causing Anthroponotic Cutaneous Leishmaniasis in Southern Iran by PCR-RFLP of Kinetoplastid DNA.Biomed Res Int. 2018 Jun 6;2018:6049198. doi: 10.1155/2018/6049198. eCollection 2018. Biomed Res Int. 2018. PMID: 29984240 Free PMC article.
-
Whole genome sequencing of Trypanosoma cruzi field isolates reveals extensive genomic variability and complex aneuploidy patterns within TcII DTU.BMC Genomics. 2018 Nov 13;19(1):816. doi: 10.1186/s12864-018-5198-4. BMC Genomics. 2018. PMID: 30424726 Free PMC article.
References
-
- Herwaldt BL (1999) Leishmaniasis. Lancet 354: 1191–1199. - PubMed
-
- Sacks D, Kamhawi S (2001) Molecular aspects of parasite-vector and vector-host interactions in leishmaniasis. Annu Rev Microbiol 55: 453–483. - PubMed
-
- Kaye P, Scott P (2011) Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol 9: 604–615. - PubMed
-
- Tibayrenc M, Ayala FJ (2002) The clonal theory of parasitic protozoa: 12 years on. Trends in Parasitology 18: 405–410. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

