Immunogenicity of influenza vaccine in colorectal cancer patients
- PMID: 24454003
- PMCID: PMC3893328
- DOI: 10.4143/crt.2013.45.4.303
Immunogenicity of influenza vaccine in colorectal cancer patients
Abstract
Purpose: Although influenza is regarded as a major cause of morbidity and mortality in immunocompromised patients, vaccine coverage remains poor. We evaluated the immunogenicity of influenza vaccines in colorectal cancer patients.
Materials and methods: In this study, 40 colorectal cancer patients who received an influenza vaccine at the Korea Cancer Center Hospital during the 2009-2010 and 2010-2011 influenza seasons were analyzed. The blood samples were collected at prevaccination and 30 days post vaccination, and antibody titers were measured using the hemagglutination-inhibition tests.
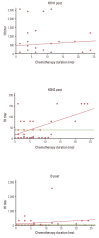
Results: In the 2009-2011 season, the seroprotection rate for H1N1 (94.7%) was significantly higher than that for H3N2 (42.1%) and B (47.3%). The seroconversion rate was 52.6%, 26.3%, and 36.8% for H1N1, H3N2, and B, respectively. Fold increase of geometric mean titer (MFI) was 3.86, 1.49, and 3.33 for H1N1, H3N2, and B, respectively. In the 2010-2011 season, the seroprotection rate for H1N1 (57.1%) was significantly higher than that for H3N2 (52.4%) and B (38.1%). The seroconversion rate was 52.4%, 47.6% and 33.3% for H1N1, H3N2, and B, respectively. MFI was 12.29, 3.62 and 4.27 for H1N1, H3N2, and B, respectively.
Conclusion: Our study cohort showed an acceptable immune response to an influenza vaccine without significant adverse effects, supporting the recommendation for annual influenza vaccination in colorectal cancer patients.
Keywords: Colorectal neoplasms; Hemagglutination inhibition tests; Human influenza; Influenza vaccines.
Conflict of interest statement
Conflict of interest relevant to this article was not reported.
Figures
Similar articles
-
Quadrivalent Influenza Vaccine-Induced Antibody Response and Influencing Determinants in Patients ≥ 55 Years of Age in the 2018/2019 Season.Int J Environ Res Public Health. 2019 Nov 14;16(22):4489. doi: 10.3390/ijerph16224489. Int J Environ Res Public Health. 2019. PMID: 31739554 Free PMC article.
-
Immunization with trivalent inactivated influenza vaccine in partially immunized toddlers.Pediatrics. 2006 Sep;118(3):e579-85. doi: 10.1542/peds.2006-0201. Pediatrics. 2006. PMID: 16950949 Clinical Trial.
-
Influenza vaccine immunogenicity in 6- to 23-month-old children: are identical antigens necessary for priming?Pediatrics. 2006 Sep;118(3):e570-8. doi: 10.1542/peds.2006-0198. Pediatrics. 2006. PMID: 16950948 Clinical Trial.
-
Immunogenicity and Safety of Reduced-Dose Intradermal vs Intramuscular Influenza Vaccines: A Systematic Review and Meta-analysis.JAMA Netw Open. 2021 Feb 1;4(2):e2035693. doi: 10.1001/jamanetworkopen.2020.35693. JAMA Netw Open. 2021. PMID: 33560425 Free PMC article.
-
Immunogenicity of influenza vaccine in elderly people: a systematic review and meta-analysis of randomized controlled trials, and its association with real-world effectiveness.Hum Vaccin Immunother. 2020 Nov 1;16(11):2680-2689. doi: 10.1080/21645515.2020.1747375. Epub 2020 Apr 29. Hum Vaccin Immunother. 2020. PMID: 32347787 Free PMC article.
Cited by
-
Immunogenicity of trivalent influenza vaccine in patients with lung cancer undergoing anticancer chemotherapy.Hum Vaccin Immunother. 2017 Mar 4;13(3):543-550. doi: 10.1080/21645515.2016.1246094. Epub 2016 Nov 7. Hum Vaccin Immunother. 2017. PMID: 27820665 Free PMC article.
-
Quadrivalent Influenza Vaccine-Induced Antibody Response and Influencing Determinants in Patients ≥ 55 Years of Age in the 2018/2019 Season.Int J Environ Res Public Health. 2019 Nov 14;16(22):4489. doi: 10.3390/ijerph16224489. Int J Environ Res Public Health. 2019. PMID: 31739554 Free PMC article.
-
Immunogenicity of Influenza Vaccination in Patients With Cancer.Am J Clin Oncol. 2018 Mar;41(3):248-253. doi: 10.1097/COC.0000000000000257. Am J Clin Oncol. 2018. PMID: 26669741 Free PMC article. Clinical Trial.
References
-
- Whitley RJ, Monto AS. Prevention and treatment of influenza in high-risk groups: children, pregnant women, immunocompromised hosts, and nursing home residents. J Infect Dis. 2006;194(Suppl 2):S133–S138. - PubMed
-
- Ljungman P, Andersson J, Aschan J, Barkholt L, Ehrnst A, Johansson M, et al. Influenza A in immunocompromised patients. Clin Infect Dis. 1993;17:244–247. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources