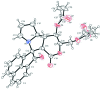
(1S,1'S,2'R,4a'S,9a'S,9b'R)-1'-Acet-yloxy-2,4'-dioxo-2',4',4a',7',8',9',9a',9b'-octa-hydro-1'H,2H-spiro-[ace-naphthyl-ene-1,5'-pyrano[4,3-a]pyrrolizin]-2'-ylmethyl acetate
- PMID: 24454054
- PMCID: PMC3884278
- DOI: 10.1107/S1600536813026111
(1S,1'S,2'R,4a'S,9a'S,9b'R)-1'-Acet-yloxy-2,4'-dioxo-2',4',4a',7',8',9',9a',9b'-octa-hydro-1'H,2H-spiro-[ace-naphthyl-ene-1,5'-pyrano[4,3-a]pyrrolizin]-2'-ylmethyl acetate
Abstract
In the title compound C26H25NO7, the mean plane through the lactone-substituted ring of the pyrrolizidine moiety forms dihedral angles of 78.46 (6) and 58.28 (8)° with the ace-naphthyl-ene moiety and the sugar based-lactone ring, respectively. The sum of the angles at the the N atom of the pyrrolizidine ring (335.0°) is in accordance with sp (3) hybridization. Some atoms of the acetate group are disordered and were refined using a split model [occupancy ratio 0.673 (10):0.327 (10)].
Figures
References
-
- Altomare, A., Cascarano, G., Giacovazzo, C. & Guagliardi, A. (1993). J. Appl. Cryst. 26, 343–350.
-
- Boido, A., Vazzana, I. & Sparatore, F. (1994). Il Farmaco, 49, 97–104. - PubMed
-
- Bruker (2004). APEX2, SAINT, XPREP and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Cravotto, G., Giovenzana, G. B., Pilati, T., Sisti, M. & Palmisano, G. (2001). J. Org. Chem. 66, 8447–8453. - PubMed
-
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous