2-Allyl-7-nitro-2H-indazole
- PMID: 24454056
- PMCID: PMC3884280
- DOI: 10.1107/S1600536813026743
2-Allyl-7-nitro-2H-indazole
Abstract
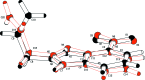
The asymmetric unit of the title compound, C10H9N3O2, contains two independent mol-ecules linked by a C-H⋯N hydrogen bond. Each mol-ecule has a similar conformation, being built up from fused five- and six-membered rings, each linked to an ally and nitro group, respectively. The indazole ring system makes dihedral angles of 2.7 (2) and 2.2 (2)°, respectively, with the plane through the nitro group. The allyl group is nearly perpendicular to the indazole system, as indicated by the N-N-C-C torsion angles of -75.3 (2) and -82.2 (2)°, this being the most important difference between the conformations of the two mol-ecules. In the crystal, mol-ecules are linked by C-H⋯O and π-π [inter-centroid distance = 3.6225 (8) Å] inter-actions to form a three-dimensional network.
Figures

References
-
- Baraldi, P. G., Balbonic, G., Pavani, M. G., Spalluto, G., Tabrizi, M. A., Clercq, E. D., Balzarini, J., Bando, T., Sugiyama, H. & Romagnoli, R. (2001). J. Med. Chem. 44, 2536–2543. - PubMed
-
- Bruker (2009). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
