(E)-2-[2-(3-Nitro-phen-yl)ethen-yl]quinolin-8-ol
- PMID: 24454092
- PMCID: PMC3884316
- DOI: 10.1107/S1600536813027815
(E)-2-[2-(3-Nitro-phen-yl)ethen-yl]quinolin-8-ol
Abstract
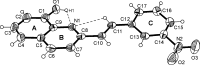
In the title compound, C17H12N2O3, the mean planes of the benzene ring and the quinoline moiety are inclined to one another by 11.0 (1)°. The nitro substituent is twisted at an angle of 7.9 (2)° with respect to the attached benzene ring. Intra-molecular O-H⋯N and C-H⋯N hydrogen bonds occur. The crystal is constructed of mol-ecular stacks without involvement of π-stacking inter-actions, but showing inter-stack association via O-H⋯O and C-H⋯O hydrogen bonding. Thus, the supramolecular architecture of the crystal results from stacked molecules stabilized by hydrogen bonding between the stacks.
Figures
References
-
- Albrecht, M., Fiege, M. & Osetska, O. (2008). Coord. Chem. Rev. 252, 812–824.
-
- Bruker (2007). SAINT-NT, SMART and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Desiraju, G. R. & Steiner, T. (1999). The Weak Hydrogen Bond in Structural Chemistry and Biology, ch. 2. Oxford University Press.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
