3-(1,3-Di-phenyl-propan-2-yl)-4-methyl-6-phenyl-isoxazolo[3,4-d]pyridazin-7(6H)-one
- PMID: 24454112
- PMCID: PMC3884336
- DOI: 10.1107/S160053681302802X
3-(1,3-Di-phenyl-propan-2-yl)-4-methyl-6-phenyl-isoxazolo[3,4-d]pyridazin-7(6H)-one
Abstract
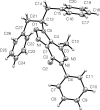
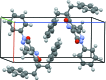
In the title compound, C27H23N3O2, the geminal benzyl groups branching out from the methine adjacent to the isoxazole group are both syn-oriented to the methyl group of the pyridazinone moiety, as reflected by C-C distances of 3.812 (2) and 4.369 (2) Å between the methyl carbon and the nearest ring carbon of each benzyl group. This kind of conformation is retained in CDCl3 solution, as evidenced by distinct phenyl-shielding effects on the (1)H NMR signals of the methyl H atoms. The isoxazolo[3,4-d]pyridazin ring system is virtually planar (r.m.s. deviation from planarity = 0.031 Å), but the N-bonded phenyl group is inclined to the former by an ring-ring angle of 55.05 (3)°. In the crystal, the T-shaped mol-ecules are arranged in an inter-locked fashion, forming rod-like assemblies along [10-1]. The mol-ecules are held together by unremarkable weak C-H⋯N, C-H⋯O and C-H⋯π inter-actions (C-O,N,C > 3.4 A), while significant π-π-stacking inter-actions are absent.
Figures
References
-
- Bruker (2012). SMART, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Chimichi, S., Ciciani, G., Dal Piaz, V., De Sio, F., Sarti-Fantoni, P. & Torroba, T. (1986). Heterocycles, 24, 3467–3471.
-
- Dal Piaz, V., Ciciani, G. & Chimichi, S. (1985). Heterocycles, 23, 365–369.
-
- Dal Piaz, V., Pinzauti, S. & Lacrimini, P. (1975). J. Heterocycl. Chem. 13, 409–410.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
