3-[1-(4-Bromo-phen-yl)eth-oxy]-2,2,5-trimethyl-4-phenyl-3-aza-hexa-ne
- PMID: 24454232
- PMCID: PMC3885056
- DOI: 10.1107/S1600536813029966
3-[1-(4-Bromo-phen-yl)eth-oxy]-2,2,5-trimethyl-4-phenyl-3-aza-hexa-ne
Abstract
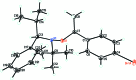
The title compound, C22H30BrNO, is an alk-oxy-amine compound, an effective initiator in nitroxide-mediated free radical polymerization. It was prepared as a mixture of two diasteromers; the crystal for the X-ray analysis showed one of these as a pair of R,S and S,R enanti-omers. The tert-butyl and isopropyl groups are in an almost anti conformation in the crystal [C-N-C-C torsion angle = -168.8 (1)°], and the methyl group of the ethoxy group is in an approximate anti relationship to the tert-butyl group. The dihedral angle between the phenyl and benzene rings is 33.12 (7)°. The Br atom is disordered over two positions, with occupancies of 0.9139 (16) and 0.0861 (16). In the crystal, weak C-H⋯Br contacts link the mol-ecules into chains along [-110].
Figures

References
-
- Agilent (2012). CrysAlis PRO and CrysAlis RED Agilent Technologies, Yarnton, England.
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
-
- Benoit, D., Chaplinski, V., Braslau, R. & Hawker, C. J. (1999). J. Am. Chem. Soc. 121, 3904–3920.
-
- Clark, R. C. & Reid, J. S. (1995). Acta Cryst. A51, 887–897.
-
- Kaul, E., Senkovskyy, V., Tkachov, R., Bocharova, V., Komber, H., Stamm, M. & Kiriy, A. (2010). Macromolecules, 43, 77–81.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
